Identification of CIPK Family in Rice and qRT-PCR Analysis on OsCIPK5 Induced by Magnaporthe oryzae
-
摘要:
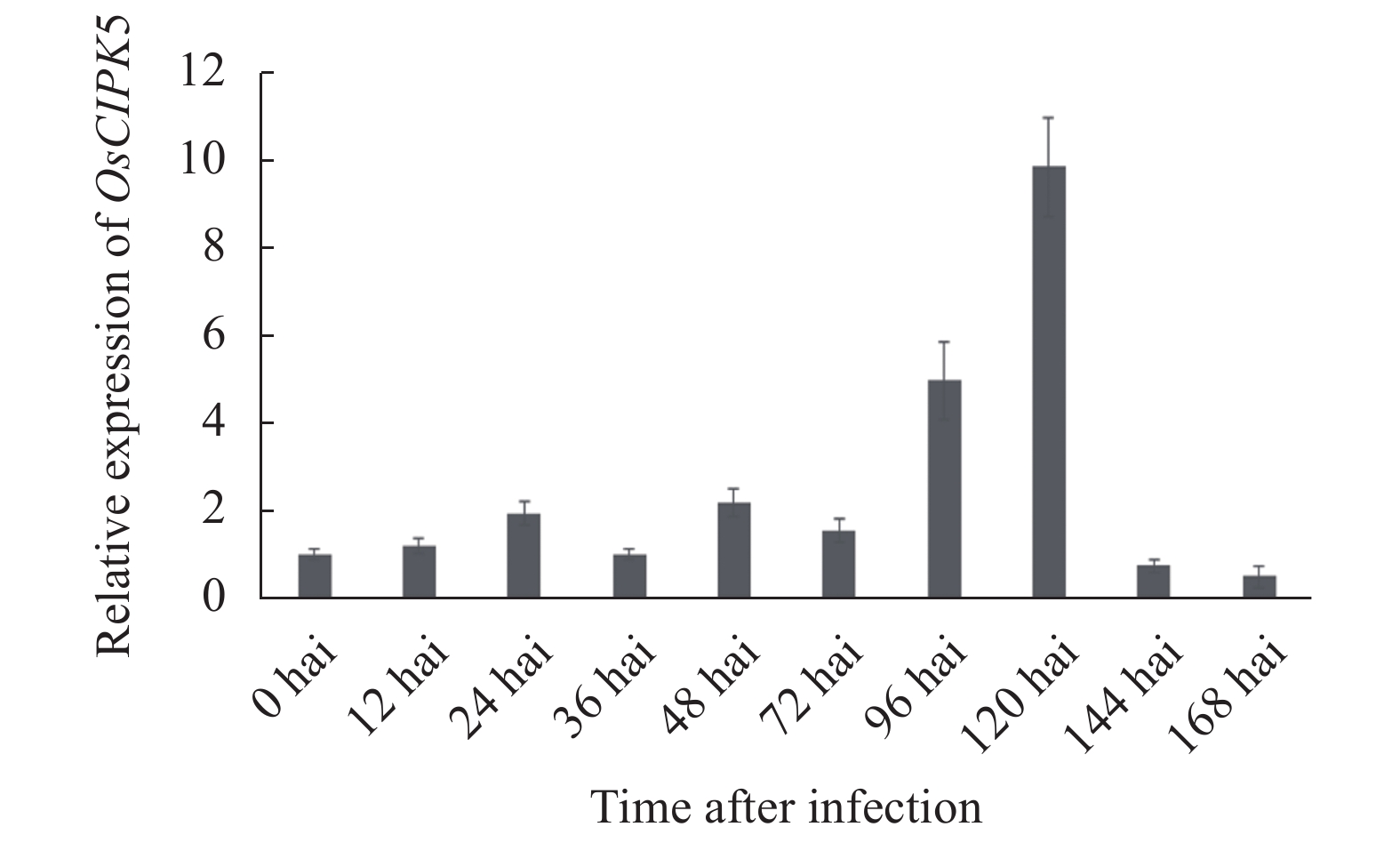
目的 植物通过启动一系列信号传导过程来应对外部环境,这些过程通常涉及多种蛋白激酶,包括钙调神经磷酸酶B样蛋白互作激酶(calcineurin B-like protein-interacting protein kinases, CIPKs)。为更加清晰全面地了解水稻CIPK基因家族,本研究根据最新的基因组测序数据对水稻基因组中的CIPKs进行了鉴定。 方法 通过探讨拟南芥和水稻中CIPKs蛋白家族的结构特点,结合生物信息学和qRT-PCR技术系统分析水稻中CIPKs家族蛋白的结构。结合转录组数据,比较了粳稻云引受稻瘟病菌诱导后的表达情况。 结果 根据最新的水稻基因组数据,鉴定出31个水稻OsCIPK基因。系统发育树分析结果表明,31个OsCIPK基因可分为5个亚家族,这些亚家族具有不同的外显子-内含子和UTR的结构特点。从广谱抗稻瘟病品种粳稻云引受稻瘟病菌诱导的基因表达谱的趋势聚类中筛选出了OsCIPK5基因并对其进行了表达分析,结果表明,云引中OsCIPK5基因受稻瘟病菌的诱导表达。 结论 内含子缺失和片段重复在水稻OsCIPK基因家族的扩展中起到重要作用,同时OsCIPK5受到稻瘟病菌的诱导表达。 Abstract:Objective The family of calcineurin B-like protein-interacting protein kinase genes (CIPKs) in rice was studied using the latest sequencing data to further understand the signal transduction involving a variety of kinases in plants in respond to environmental changes. Methods The structures of CIPKs in Arabidopsis thaliana and rice were analyzed. Combining the bioinformatics and qRT-PCR technology, expressions of the CIPK familie and that of the genes induced by M. grisea in Japonica rice cv. 'Yunyin' were compared. Results A total of 31 OsCIPK genes were identified in the rice genome databank. The phylogenetic tree analysis showed that these 31 OsCIPKs could be divided into 5 subfamilies, which had different structural characteristics of exon-intron and UTR. The expression of OsCIPK5, which was selected from a trend cluster of the gene expressiong profile of 'Yunyin' induced by M. grisea, could be induced by M. grisea. Conclusions It appeared that the intron deletion and fragment duplication played an important role in the expansion of OsCIPK family in rice, and that OsCIPK5 expression was induced in 'Yunyin' by M. grisea. -
图 2 水稻CIPK基因的系统发育分类、基因结构及保守蛋白基序的特征
注:① a为系统发育树,b为蛋白基序结构特征,c为基因结构;② OsCIPK系统发育树分为五组:A、B、C、D和E,用不同的颜色标出;③b图标尺为氨基酸长度,c图标尺为核苷酸长度;④绿色方框表示UTR,黄色方框表示CDS,黑线表示内含子,其他颜色方框代表保守结构域。
Figure 2. Phylogenetic relationships, gene structure and architecture of conserved protein motifs in CIPKs from rice
Note: ① a, phylogenetic tree; b, protein motif structure; and c, gene structure. ② OsCIPK phylogenetic tree was divided into 5 groups -A, B, C, D, and E- shown in different colors. ③ The ruler of b is for amino acid length; and the ruler of c is for nucleotide length. ④ Green box shows UTR; yellow box shows CDs; black line shows introns; and boxes of other colors show conservative domains.
表 1 OsCIPK5和Actin的引物序列
Table 1. The primer sequence of OsCIPK5 and Actin
基因 Genes q-F q-R OsCIPK5 CAGAGCGTCGCCATCAAGGTC GTGACAGAAGTCCACAGCCCCTATC Actin CCTCGTCTGCGATAATGGAACTG CCCTGGGCGCATCATCTC 表 2 水稻基因组CIPK家族的特征
Table 2. Characteristics of CIPK family in rice genome
基因名称
Name of gene转录本ID(RAP-DB)
Transcriptome染色体位置
Locus of chromosome氨基酸长度
Length of amino acid等电点(PI)
Isoelectric point分子量(WM)
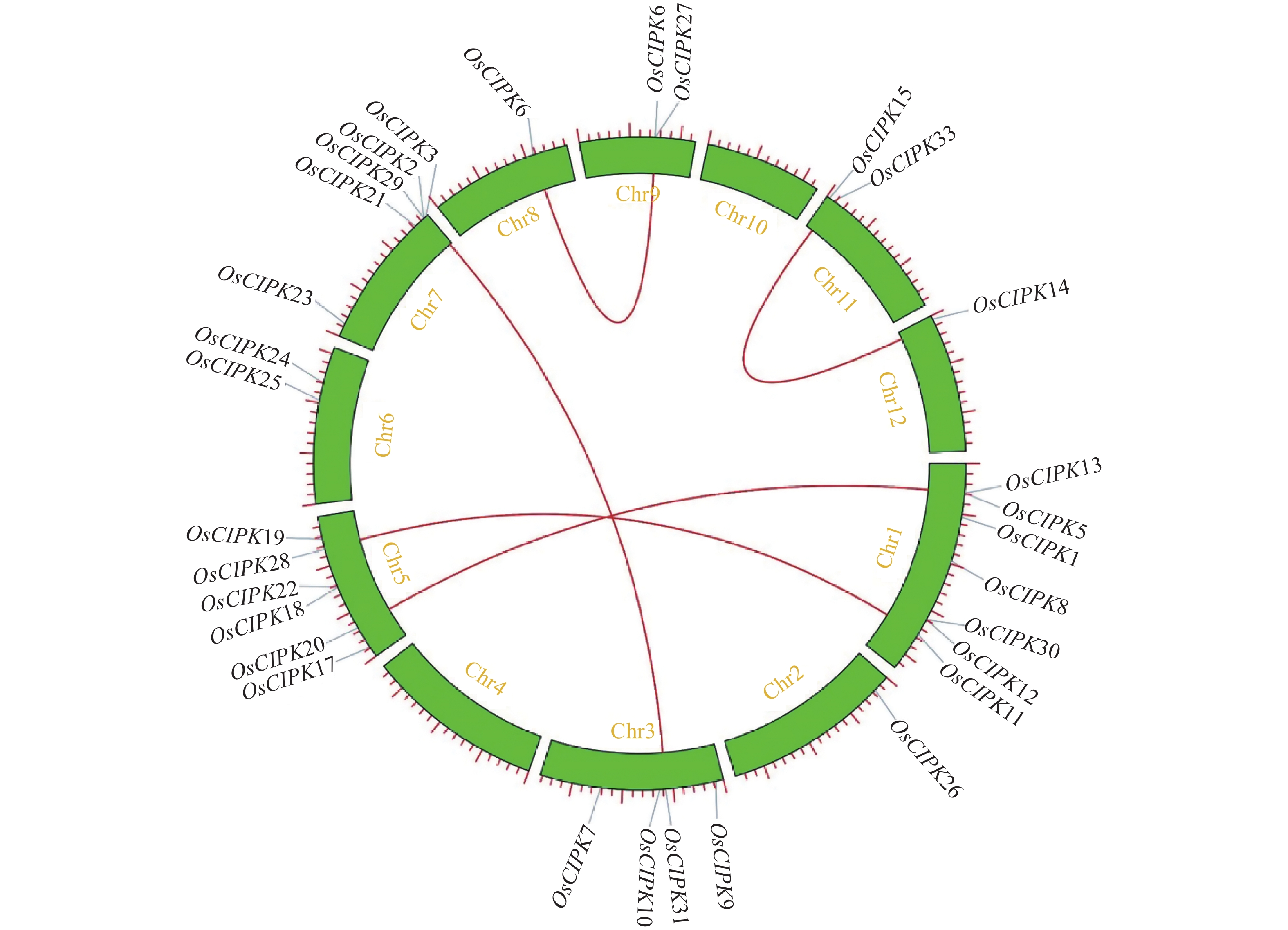
Weight of molecular/kDaOsCIPK1 Os01t0292200-01 chr1:10622951-10627532 462 6.26 52.20 OsCIPK2 Os07t0678600-01 chr7:28726817-28729527 444 9.18 50.27 OsCIPK3 Os07t0687000-01 chr7:29191197-29194187 446 6.9 50.95 OsCIPK5 Os01t0206700-03 chr1:5809589-5811389 462 9.28 51.97 OsCIPK6 Os08t0441100-01 chr8:21467453-21468593 305 5.44 32.48 OsCIPK7 Os03t0634400-01 chr3:24226372-24227930 448 9.35 48.37 OsCIPK8 Os01t0536000-01 chr1:19469262-19477590 447 6.45 50.60 OsCIPK9 Os03t0126800-00 chr3:1515355-1518947 479 8.31 53.78 OsCIPK10 Os03t0339900-01 chr3:12630705-12635182 452 9.01 51..49 OsCIPK11 Os01t0824600-01 chr1:35228913-35231725 503 8.48 56.56 OsCIPK12 Os01t0759400-02 chr1:31943529-31948580 541 8.3 59.81 OsCIPK13 Os01t0206300-00 chr1:5799607-5801142 512 8.05 56.01 OsCIPK14 Os12t0113500-01 chr12:679079-682143 440 9.44 50.32 OsCIPK15 Os11t0113700-01 chr11:630585-633622 435 9.53 49.70 OsCIPK16 Os09t0418000-03 chr9:15009182-15010716 457 8.84 50.50 OsCIPK17 Os05t0136200-01 chr5:2113595-2117110 455 6.93 50.92 OsCIPK18 Os05t0332300-01 chr5:15556085-15560404 458 8.84 51.50 OsCIPK19 Os05t0514200-01 chr5:25489349-25491319 509 7.26 56.91 OsCIPK20 Os05t0208100-01 chr5:6701053-6702933 467 8.29 51.35 OsCIPK21 Os07t0637000-01 chr7:26464444-26468858 445 7.96 50.10 OsCIPK22 Os05t0334750-00 chr5:15643309-15644732 452 8.03 49.32 OsCIPK23 Os07t0150700-01 chr7:2643540-2647773 451 9.23 50.71 OsCIPK24 Os06t0606000-01 chr6:24038959-24044785 454 8.52 50.94 OsCIPK25 Os06t0543400-01 chr6:20490388-20492271 515 8.78 56.98 OsCIPK26 Os02t0161000-01 chr2:3283264-3286366 494 9.13 55.91 OsCIPK27 Os09t0418500-00 chr9:15027311-15034300 809 5.53 87.30 OsCIPK28 Os05t0476350-00 chr5:23430891-23432198 436 9.32 49.33 OsCIPK29 Os07t0678300-01 chr7:28710749-28712470 444 8.69 48.21 OsCIPK30 Os01t0759200-01 chr1:31938559-31940184 477 9.34 53.56 OsCIPK31 Os03t0319400-01 chr3:11526273-11530306 450 7.98 50.95 OsCIPK33 Os11t0134300-01 chr11:1605182-1609130 406 6.13 46.60 表 3 水稻基因组中的直系同源CIPK基因对的Ka/Ks比值
Table 3. Ka/Ks value of orthologous CIPK gene pairs in rice genome
直系同源CIPK基因对
The homologous CIPK gene pairKa Ks Ka/Ks OsCIPK31/OsCIPK3 0.113 65 1.115 82 0.101 85 OsCIPK15/OsCIPK14 0.004 17 0.035 81 0.116 45 OsCIPK11/OsCIPK28 0.161 62 0.880 47 0.183 55 OsCIPK5/OsCIPK20 0.221 63 1.297 74 0.170 78 OsCIPK6/OsCIPK27 0.348 63 0.529 36 0.658 59 -
[1] BAUM G, LONG J C, JENKINS G I, et al. Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca2+ [J]. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96(23): 13554−13559. doi: 10.1073/pnas.96.23.13554 [2] EVANS N H, MCAINSH M R, HETHERINGTON A M. Calcium oscillations in higher plants [J]. Current Opinion in Plant Biology, 2001, 4(5): 415−420. doi: 10.1016/S1369-5266(00)00194-1 [3] KNIGHT H, KNIGHT M R. Abiotic stress signalling pathways: specificity and cross-talk [J]. Trends in Plant Science, 2001, 6(6): 262−267. doi: 10.1016/S1360-1385(01)01946-X [4] MACROBBIE E A C. ABA activates multiple Ca2+ fluxes in stomatal guard cells, triggering vacuolar K+(Rb+) release [J]. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(22): 12361−12368. doi: 10.1073/pnas.220417197 [5] SANDERS D, BROWNLEE C, HARPER J F. Communicating with calcium [J]. The Plant Cell, 1999, 11(4): 691. doi: 10.1105/tpc.11.4.691 [6] CHENG S H, WILLMANN M R, CHEN H C, et al. Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family [J]. Plant Physiology, 2002, 129(2): 469−485. doi: 10.1104/pp.005645 [7] HARMON A C, GRIBSKOV M, GUBRIUM E, et al. The CDPK superfamily of protein kinases [J]. New Phytologist, 2001, 151(1): 175−183. doi: 10.1046/j.1469-8137.2001.00171.x [8] LUAN S, RODRIGUEZCONCEPCION M, YALOVSKY S, et al. Calmodulins and calcineurin B-like proteins: calcium sensors for specific signal response coupling in plants [J]. The Plant Cell, 2002, 14: S389−400. doi: 10.1105/tpc.001115 [9] SNEDDEN W A, FROMM H. Calmodulin as a versatile calcium signal transducer in plants [J]. New Phytologist, 2001, 151(1): 35−66. doi: 10.1046/j.1469-8137.2001.00154.x [10] ZIELINSKI R E. Calmodulin and calmodulin-binding proteins in plants [J]. Annual Review of Plant Physiology and Plant Molecular Biology, 1998, 49(1): 697−725. doi: 10.1146/annurev.arplant.49.1.697 [11] LIU J, ISHITANI M, HALFTER U, et al. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance [J]. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(7): 3730−3734. doi: 10.1073/pnas.97.7.3730 [12] ISHITANI M, LIU J P, HALFTER U, et al. SOS3 function in plant salt tolerance requires N-myristoylation and calcium binding [J]. The Plant Cell, 2000, 12(9): 1667−1677. doi: 10.1105/tpc.12.9.1667 [13] SÁNCHEZ-BARRENA M J, MARTÍNEZ-RIPOLL M, ZHU J K, et al. The structure of the Arabidopsis thaliana SOS3: molecular mechanism of sensing calcium for salt stress response [J]. Journal of Molecular Biology, 2005, 345(5): 1253−1264. doi: 10.1016/j.jmb.2004.11.025 [14] GONG D M, GUO Y, SCHUMAKER K S, et al. The SOS3 family of calcium sensors and SOS2 family of protein kinases in Arabidopsis [J]. Plant Physiology, 2004, 134(3): 919−926. doi: 10.1104/pp.103.037440 [15] SHI H Z, ISHITANI M, KIM C, et al. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter [J]. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(12): 6896−6901. doi: 10.1073/pnas.120170197 [16] QIU Q S, GUO Y, DIETRICH M A, et al. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3 [J]. Proceedings of the National Academy of Sciences of the United States of America, 2002, 99(12): 8436−8441. doi: 10.1073/pnas.122224699 [17] QIU Q S, GUO Y, QUINTERO F J, et al. Regulation of vacuolar Na+/H+ exchange in Arabidopsis thaliana by the salt-overly-sensitive (SOS) pathway [J]. Journal of Biological Chemistry, 2004, 279(1): 207−215. doi: 10.1074/jbc.M307982200 [18] XIONG L M, SCHUMAKER K S, ZHU J K. Cell signaling during cold, drought, and salt stress [J]. The Plant Cell, 2002, 14(S1): S165−183. [19] ZHU J K. Salt and drought stress signal transduction in plants [J]. Annual Review of Plant Biology, 2002, 53(1): 247−273. doi: 10.1146/annurev.arplant.53.091401.143329 [20] KOLUKISAOGLUÜ, WEINL S, BLAZEVIC D, et al. Calcium sensors and their interacting protein kinases: genomics of the Arabidopsis and rice CBL-CIPK signaling networks [J]. Plant Physiology, 2004, 134(1): 43−58. doi: 10.1104/pp.103.033068 [21] XIANG Y, HUANG Y M, XIONG L Z. Characterization of stress-responsive CIPK genes in rice for stress tolerance improvement [J]. Plant Physiology, 2007, 144(3): 1416−1428. doi: 10.1104/pp.107.101295 [22] CHEN X F, GU Z M, LIU F, et al. Molecular analysis of rice CIPKs involved in both biotic and abiotic stress responses [J]. Rice Science, 2011, 18(1): 1−9. doi: 10.1016/S1672-6308(11)60001-2 [23] HOLUB E B. The arms race is ancient history in Arabidopsis, the wildflower [J]. Nature Reviews Genetics, 2001, 2(7): 516−527. doi: 10.1038/35080508 [24] YU Y H, XIA X L, YIN W L, et al. Comparative genomic analysis of CIPK gene family in Arabidopsis and Populus [J]. Plant Growth Regulation, 2007, 52(2): 101−110. doi: 10.1007/s10725-007-9165-3 [25] KANWAR P, SANYAL S K, TOKAS I, et al. Comprehensive structural, interaction and expression analysis of CBL and CIPK complement during abiotic stresses and development in rice [J]. Cell Calcium, 2014, 56(2): 81−95. doi: 10.1016/j.ceca.2014.05.003 [26] ROY S W, PENNY D. Patterns of intron loss and gain in plants: intron loss–dominated evolution and genome-wide comparison of O. sativa and A. thaliana [J]. Molecular Biology and Evolution, 2007, 24(1): 171−181. [27] NURUZZAMAN M, MANIMEKALAI R, SHARONI A M, et al. Genome-wide analysis of NAC transcription factor family in rice [J]. Gene, 2010, 465(1/2): 30−44. [28] KLEIST T J, SPENCLEY A L, LUAN S. Comparative phylogenomics of the CBL-CIPK calcium-decoding network in the moss Physcomitrella, Arabidopsis, and other green lineages [J]. Frontiers in Plant Science, 2014(5): 187. [29] ZHANG H F, YANG B, LIU W Z, et al. Identification and characterization of CBL and CIPK gene families in canola (Brassica napus L.) [J]. BMC Plant Biology, 2014, 14(1): 8. doi: 10.1186/1471-2229-14-8 [30] KUDLA J, BATISTIČ O, HASHIMOTO K. Calcium signals: the lead currency of plant information processing [J]. The Plant Cell, 2010, 22(3): 541−563. doi: 10.1105/tpc.109.072686 [31] HU H C, WANG Y Y, TSAY Y F. AtCIPK8, a CBL‐interacting protein kinase, regulates the low-affinity phase of the primary nitrate response [J]. Plant Journal, 2009, 57(2): 264−278. doi: 10.1111/j.1365-313X.2008.03685.x [32] CHUNG E, PARK J M, OH S K, et al. Molecular and biochemical characterization of the Capsicum annuum calcium-dependent protein kinase 3 (CaCDPK3) gene induced by abiotic and biotic stresses [J]. Planta, 2004, 220(2): 286−295. doi: 10.1007/s00425-004-1372-9 [33] LUDWIG A A, SAITOH H, FELIX G, et al. Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants [J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(30): 10736−10741. doi: 10.1073/pnas.0502954102 [34] FROHNMEYER H, LOYALL L, BLATT M R, et al. Millisecond UV-B irradiation evokes prolonged elevation of cytosolic-free Ca2+ and stimulates gene expression in transgenic parsley cell cultures [J]. Plant Journal, 1999, 20(1): 109−117. doi: 10.1046/j.1365-313X.1999.00584.x [35] TREWAVAS A J, MALHÓ R. Ca2+ signalling in plant cells: the big network! [J]. Current Opinion in Plant Biology, 1998, 1(5): 428−443. doi: 10.1016/S1369-5266(98)80268-9 [36] TRAN P O T, HINMAN L E, UNGER G M, et al. A wound-induced[Ca2+]i increase and its transcriptional activation of immediate early genes is important in the regulation of motility [J]. Experimental Cell Research, 1999, 246(2): 319−326. doi: 10.1006/excr.1998.4239 [37] YANG W Q, KONG Z S, OMO-IKERODAH E, et al. Calcineurin B-like interacting protein kinase OsCIPK23 functions in pollination and drought stress responses in rice (Oryza sativa L.) [J]. Journal of Genetics and Genomics, 2008, 35(9): 531−543. doi: 10.1016/S1673-8527(08)60073-9 [38] OHBA H, STEWARD N, KAWASAKI S, et al. Diverse response of rice and maize genes encoding homologs of WPK4, an SNF1-related protein kinase from wheat, to light, nutrients, low temperature and cytokinins [J]. Molecular and General Genetics, 2000, 263(2): 359−366. doi: 10.1007/s004380051179 [39] KIM K N, LEE J S, HAN H E, et al. Isolation and characterization of a novel rice Ca2+-regulated protein kinase gene involved in responses to diverse signals including cold, light, cytokinins, sugars and salts [J]. Plant Molecular Biology, 2003, 52(6): 1191−1202. doi: 10.1023/B:PLAN.0000004330.62660.a2 [40] LEE K W, CHEN P W, LU C A, et al. Coordinated responses to oxygen and sugar deficiency allow rice seedlings to tolerate flooding [J]. Science Signaling, 2009, 2(91): ra61. [41] HUANG F, LIAN L, HE W, et al. Genome-wide profiling of changes in gene expression in response to infection of the japonica rice variety Yunyin by Magnaporthe oryzae [J]. Molecular Breeding, 2014, 34(4): 1965−1974. doi: 10.1007/s11032-014-0155-7 [42] DENG X M, HU W, WEI S Y, et al. TaCIPK29, a CBL-interacting protein kinase gene from wheat, confers salt stress tolerance in transgenic tobacco [J]. PLoS One, 2013, 8(7): e69881. doi: 10.1371/journal.pone.0069881 [43] CANNON S B, MITRA A, BAUMGARTEN A, et al. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana [J]. BMC Plant Biology, 2004, 4(1): 10. doi: 10.1186/1471-2229-4-10 [44] ZHU J K, LIU J P, XIONG L M. Genetic analysis of salt tolerance in Arabidopsis: evidence for a critical role of potassium nutrition [J]. The Plant Cell, 1998, 10(7): 1181−1191. doi: 10.1105/tpc.10.7.1181 [45] LI L, KIM B G, CHEONG Y H, et al. A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis [J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(33): 12625−12630. doi: 10.1073/pnas.0605129103 [46] XU J, LI H D, CHEN L Q, et al. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis [J]. Cell, 2006, 125(7): 1347−1360. doi: 10.1016/j.cell.2006.06.011 [47] MARTÍNEZ -ATIENZA J, JIANG X Y, GARCIADEBLAS B, et al. Conservation of the salt overly sensitive pathway in rice [J]. Plant Physiology, 2007, 143(2): 1001−1012. doi: 10.1104/pp.106.092635 [48] HAYASHI K, YOSHIDA H, ASHIKAWA I. Development of PCR-based allele-specific and InDel marker sets for nine rice blast resistance genes [J]. Theoretical and Applied Genetics, 2006, 113(2): 251−260. doi: 10.1007/s00122-006-0290-6 -







 下载:
下载: