Cloning and Post-harvest Physiological Deterioration Expression of MePYL12 of Cassava
-
摘要:
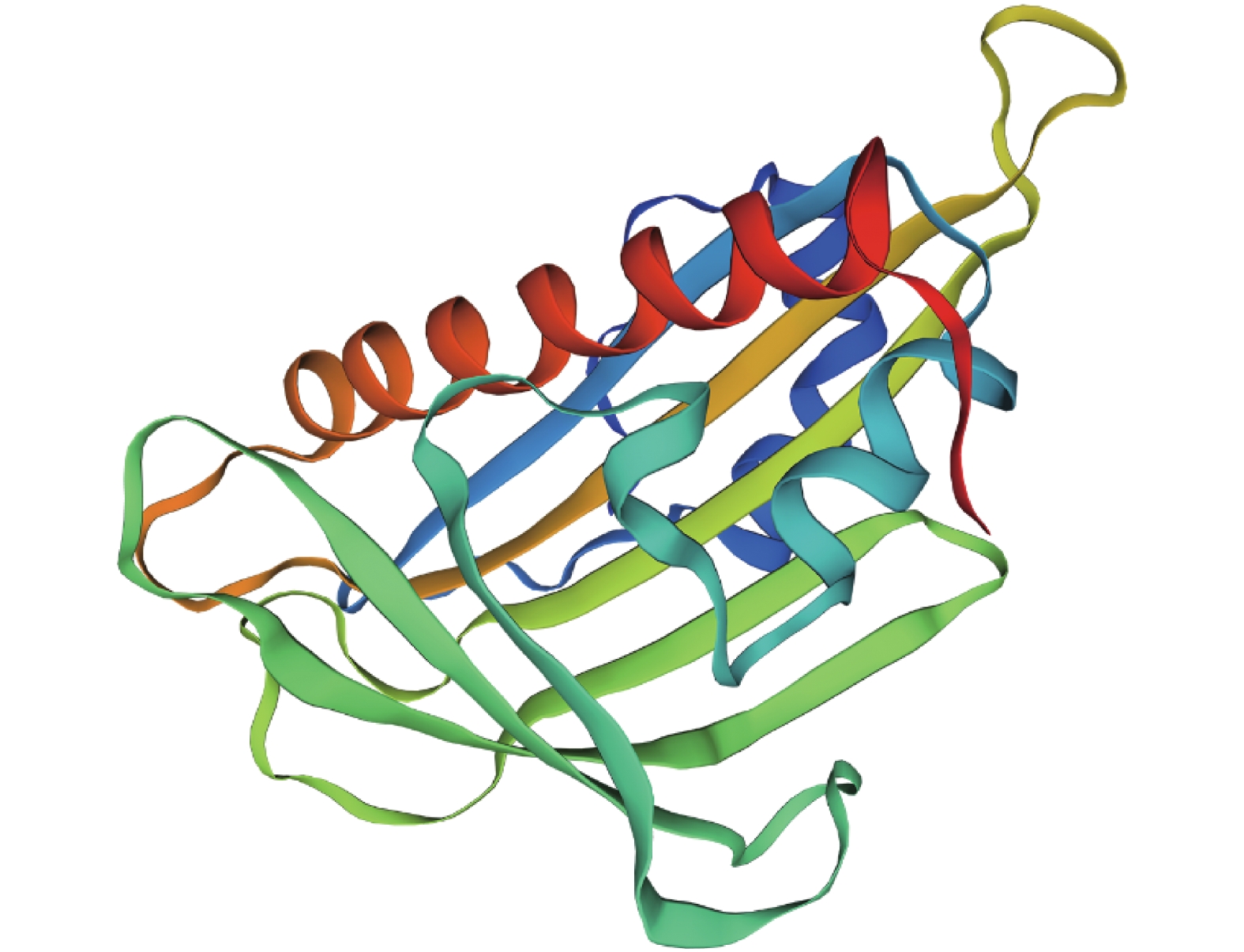
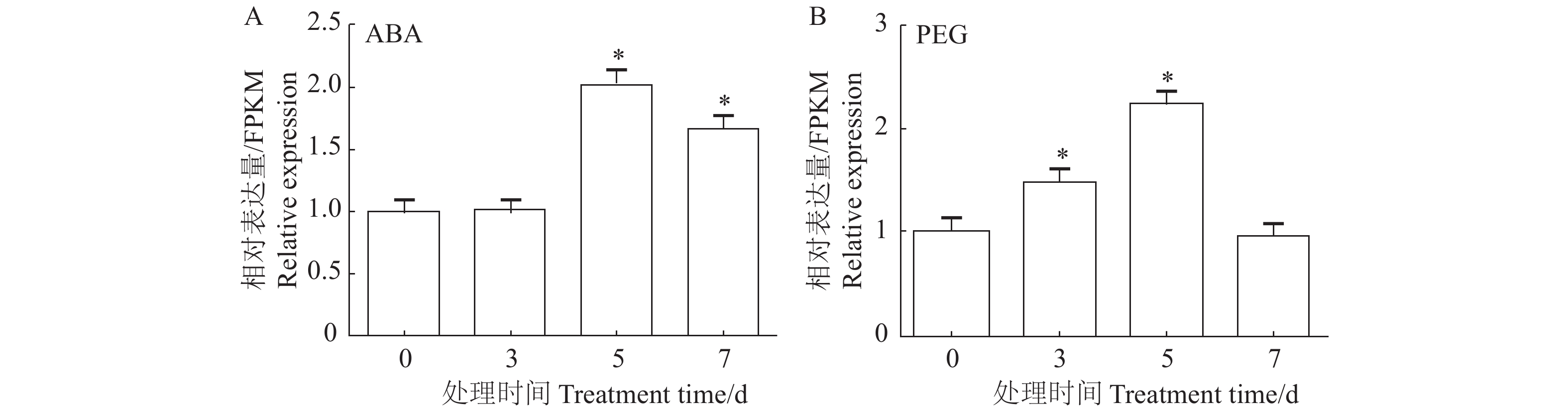
目的 脱落酸受体PYL家族基因为ABA信号通路的重要成员。研究PYL基因在木薯块根的采后生理性变质(post-harvest physiological deterioration, PPD)和非生物胁迫中的功能,能够为进一步研究ABA信号在木薯抗逆和PPD过程中的功能奠定基础。 方法 本研究从木薯品种SC124中通过RT-PCR技术克隆得到了MePYL12基因,对它的蛋白质进行相关生信分析,如遗传进化关系、结构域、蛋白质结构预测、理化性质及基因的启动子元件分析,同时对MePYL12基因在相关处理和木薯PPD过程中的表达量进行分析。 结果 (1)克隆得到的MePYL12长度为567 bp,氨基酸数量为188,理论等电点为5.55,三级结构预测显示含有典型PYL螺旋手柄结构,与蓖麻和橡胶树中PYL蛋白序列的相似性较高,达到了86.77 %和94.68 %。MePYL12基因的蛋白序列含有PYL蛋白家族的保守结构域,特别是ABA结合的区域“Latch”和“Gate”序列100%一致,这些结果表明MePYL12基因编码的蛋白质属于PYL家族成员并且高度保守。(2)10个木薯组织中的MePYL12基因表达分析显示,该基因在分生组织和叶片中的表达水平最高。(3)MePYL12基因主要的启动子元件为光应答元件(Light-responsive motifs)、干旱诱导元件(Drought-induced motif)、ABA应答元件(ABA responsive motif)等元件。(4)MePYL12基因的表达水平显著受到干旱胁迫和ABA处理诱导。在木薯块根的PPD过程也被显著诱导,在6 h达到最高,随后慢慢下降。 结论 MePYL12基因具有提高植物应对非生物胁迫能力的潜能,同时可能参与了木薯块根的PPD过程,也为后续研究相关功能奠定了基础。 Abstract:Objective Functions of PYL gene in the post-harvest physiological deterioration (PPD) and under abiotic stresses of cassava (Manihot esculenta Crantz) were studied for further research on the key component of the plant abscisic acid (ABA) signaling pathway, PYR/PYL/RCARs family. Method The PYL gene, MePYL12 was cloned from cassava SC 124 by RT-PCR. Bioinformatics was used to analyze the physicochemical properties, conserved domain, genetic evolutionary relationship, protein structure prediction, promoter elements as well as the expressions of MePYL12 in PPD and under abiotic stresses. Result (1) The full-length cDNA of MePYL12 was 567 bp encoded a polypeptide of 188 amino acids with a predicted relative molecular mass of 20.8kD and an isoelectric point of 5.55. The predicted tertiary structure contained a helix. Its multiple protein sequence alignment showed high similarities with the PYL proteins in Hevea brasiliensis (94.68%) and Ricinus communis (86.77 %). MePYL12 consisted of the conserved motifs of the PYL family, such as the ABA binding region "Latch" and "Gate" suggesting it to be a genuine member from the family and highly conserved. (2) The expressions of MePYL12 in 10 different types of cassava tissue/organ were high in the root apical meristem, shoot apical meristem, and leaf. (3) The promoter element analysis showed that the gene contained the light-responsive, drought-induced MBS, and ABA responsive ABRE motifs. (4) The expression of MePYL12 was significantly upregulated by ABA treatment and drought stress as well as during PPD that peaked in 6h followed by downregulation. Conclusion MePYL12 might participate in the PPD process improving the ability of cassava plants in dealing with abiotic stresses. Further study on the functions of MePYL12 in cassava is in order. -
Key words:
- ABA /
- MePYL12 /
- abiotic stress /
- PPD /
- expression analysis
-
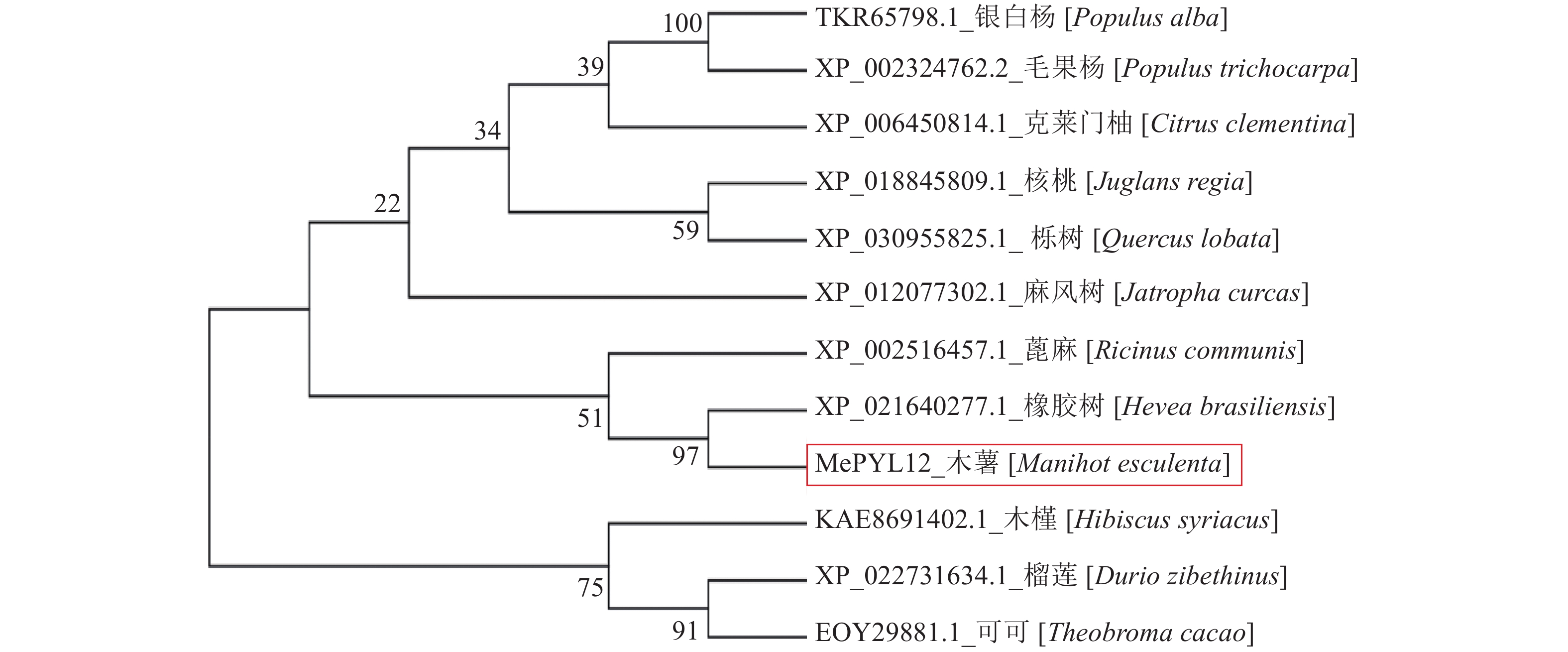
图 4 MePYL12蛋白序列的同源性比对
注:TKR65798.1银白杨[Populus alba];XP_002324762.2毛果杨[Populus trichocarpa];XP_021640277.1橡胶树[Hevea brasiliensis];XP_002516457.1蓖麻[Ricinus communis];XP_022731634.1榴莲[Durio zibethinus];EOY29881.1可可[Theobroma cacao];KAE8691402.1木瑾[Hibiscus syriacus];XP_018845809.1核桃[Juglans regia];XP_030955825.1栎树[Quercus lobata];XP_006450814.1克来门柚[Citrus clementina];XP_012077302.1麻风树[Jatropha curcas];Gate: Gate loop; Latch: Latch loop; TKR65798.1 [Populus alba]; XP_002324762.2 [Populus trichocarpa]; XP_021640277.1 [Hevea brasiliensis]; XP_002516457.1 [Ricinus communis]; XP_022731634.1 [Durio zibethinus]; EOY29881.1 [Theobroma cacao]; KAE8691402.1 [Hibiscus syriacus]; XP_018845809.1 [Juglans regia]; XP_030955825.1 [Quercus lobata]; XP_006450814.1 [Citrus clementina]; XP_012077302.1 [Jatropha curcas]; Gate: 门环;Latch: 锁环;
Figure 4. Homologous sequence alignments of MePYL12
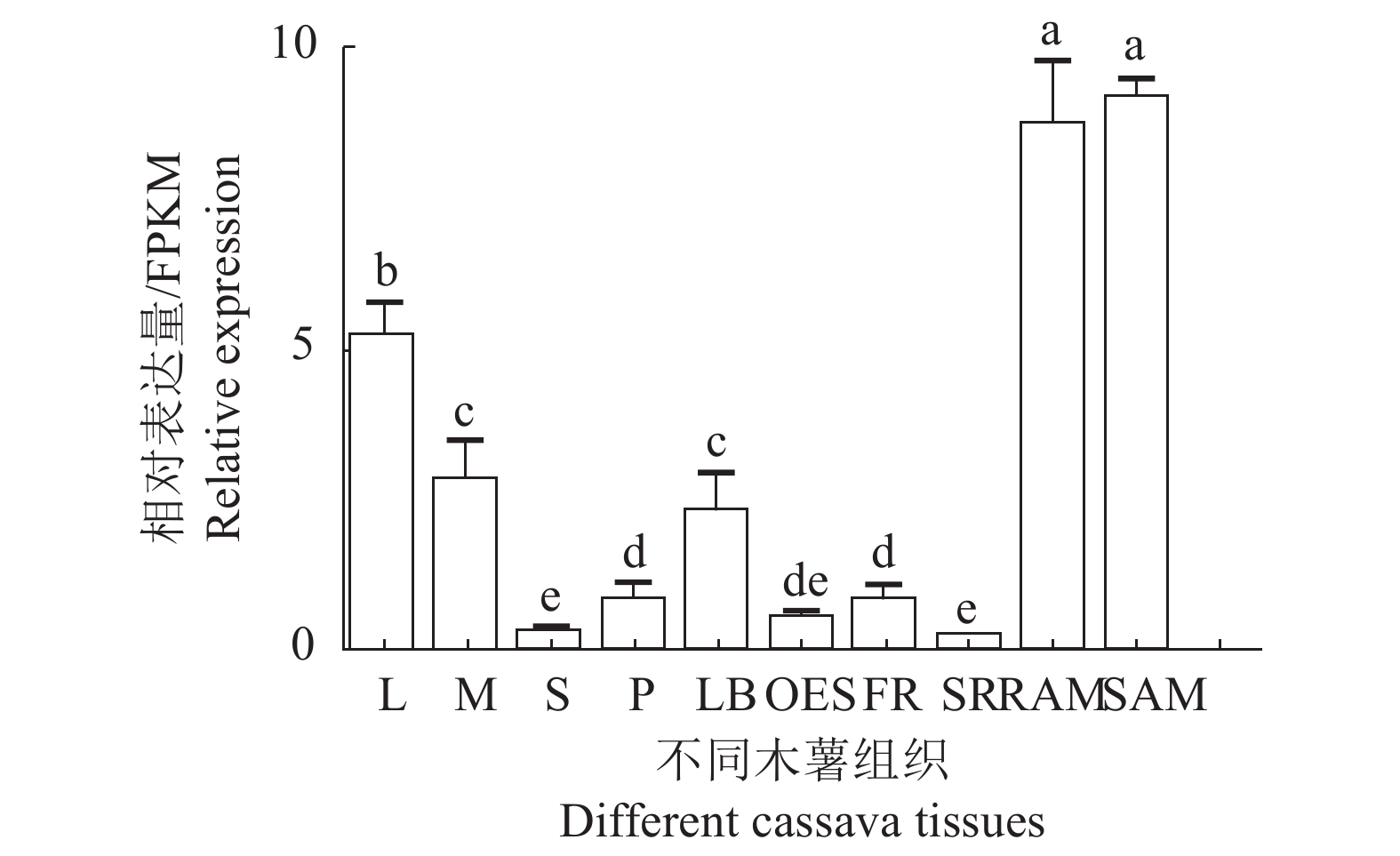
图 6 不同组织中的MePYL12表达
注:L:叶,M:中脉,S:茎,RAM:根顶端分生组织,P:叶柄,LB:侧芽,OES:分化胚组织,FR:须根,SAM:茎顶端分生组织,SR:根。差异字母则表明在Duncan's 多重比较中显著(P<0.05)。
Figure 6. Expression of MePYL12 in different tissues/organs of cassava
Note: L: leaf, M: midvein, S: stem, RAM: root apical meristem, P: petole, LB: lateral bud, OES: organized embryogenic structure, FR: fibrous root, SAM: shoot apical meristem, SR: storage root. Different letters: Significant differences based on Duncan's multiple range tests(P<0.05).
表 1 MePYL12启动子元件组成分析
Table 1. Promoter elements of MePYL12
序号
No.元件名称
Element name数目
Number功能预测
Predicted function1 光应答元件
Box 415 光应答
Light-responsive2 光应答元件
G-Box1 光应答
Light-responsive3 ABA应答元件
ABRE1 ABA应答
Abscisic acid responsive4 干旱诱导元件
MBS1 干旱诱导
Drought-inducible5 胁迫应答元件
TC-rich repeats1 防御和应激反应
Defense and stress responsiveness6 水杨酸反应元件
TCA-element1 水杨酸诱导
Salicylic acid responsiveness -
[1] RAGHAVENDRA A S, GONUGUNTA V K, CHRISTMANN A, et al. ABA perception and signalling [J]. Trends in Plant Science, 2010, 15(7): 395−401. doi: 10.1016/j.tplants.2010.04.006 [2] KRASENSKY J, JONAK C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks [J]. Journal of Experimental Botany, 2012, 63(4): 1593−1608. doi: 10.1093/jxb/err460 [3] PARK S Y, FUNG P, NISHIMURA N, et al. Abscisic acid inhibits type 2c protein phosphatases via the PYR/PYL family of start proteins [J]. Science, 2009, 324(5930): 1068−1071. [4] MA Y, SZOSTKIEWICZ I, KORTE A, et al. Regulators of PP2C phosphatase activity function as abscisic acid sensors [J]. Science, 2009, 324(5930): 1064−1068. [5] ZHANG X L, JIANG L, XIN Q, et al. Structural basis and functions of abscisic acid receptors PYLs [J]. Frontiers in Plant Science, 2015, 6: 88. [6] MIYAZONO K I, MIYAKAWA T, SAWANO Y, et al. Structural basis of abscisic acid signalling [J]. Nature, 2009, 462(7273): 609−614. doi: 10.1038/nature08583 [7] GONZALEZ-GUZMAN M, PIZZIO G A, ANTONI R, et al. Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid [J]. Plant Cell, 2012, 24(6): 2483−2496. doi: 10.1105/tpc.112.098574 [8] GONZALEZ-GUZMAN M, RODRIGUEZ L, LORENZO-ORTS L, et al. Tomato PYR/PYL/RCAR abscisic acid receptors show high expression in root, differential sensitivity to the abscisic acid agonist quinabactin, and the capability to enhance plant drought resistance [J]. Journal of Experimental Botany, 2014, 65(15): 4451−4464. doi: 10.1093/jxb/eru219 [9] LIANG C Z, LIU Y, LI Y Y, et al. Activation of ABA receptors gene GhPYL9-11A is positively correlated with cotton drought tolerance in transgenic Arabidopsis [J]. Frontiers in Plant Science, 2017, 8: 1453. doi: 10.3389/fpls.2017.01453 [10] KIM H, LEE K, HWANG H, et al. Overexpression of PYL5 in rice enhances drought tolerance, inhibits growth, and modulates gene expression [J]. Journal of Experimental Botany, 2014, 65(2): 453−464. doi: 10.1093/jxb/ert397 [11] ZHAO Y, CHAN Z L, GAO J H, et al. ABA receptor PYL9 promotes drought resistance and leaf senescence [J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(7): 1949−1954. doi: 10.1073/pnas.1522840113 [12] SANTIAGO J, RODRIGUES A, SAEZ A, et al. Modulation of drought resistance by the abscisic acid receptor PYL5 through inhibition of clade A PP2Cs [J]. Plant Journal, 2009, 60(4): 575−588. doi: 10.1111/j.1365-313X.2009.03981.x [13] CHEN Z Q, KONG L, ZHOU Y, et al. Endosperm-specific OsPYL8 and OsPYL9 act as positive regulators of the ABA signaling pathway in rice seed germination [J]. Functional Plant Biology, 2017, 44(6): 635−645. doi: 10.1071/FP16314 [14] YU J L, GE H M, WANG X K, et al. Overexpression of pyrabactin resistance-like abscisic acid receptors enhances drought, osmotic, and cold tolerance in transgenic poplars [J]. Frontiers in Plant Science, 2017, 8: 1752. doi: 10.3389/fpls.2017.01752 [15] HE Z H, ZHONG J W, SUN X P, et al. The maize ABA receptors ZmPYL8, 9, and 12 facilitate plant drought resistance [J]. Frontiers in Plant Science, 2018, 9: 422. doi: 10.3389/fpls.2018.00422 [16] 颜彦, 铁韦韦, 丁泽红, 等. 木薯MePYL8基因克隆及表达分析 [J]. 分子植物育种, 2018, 16(14):4498−4504.YAN Y, TIE W W, DING Z H, et al. Cloning and expression analysis of MePYL8 gene in cassava [J]. Molecular Plant Breeding, 2018, 16(14): 4498−4504.(in Chinese) [17] 赵思涵, 颜彦, 陈志晟, 等. 木薯PYL13基因克隆及表达分析 [J]. 南方农业学报, 2019, 50(12):2629−2637.ZHAO S H, YAN Y, CHEN Z S, et al. Cloning and expression analysis of MePYL13 gene in cassava [J]. Journal of Southern Agriculture, 2019, 50(12): 2629−2637.(in Chinese) [18] LIU S, ZAINUDDIN I M, VANDERSCHUREN H, et al. RNAi inhibition of feruloyl CoA 6'-hydroxylase reduces scopoletin biosynthesis and post-harvest physiological deterioration in cassava (Manihot esculenta Crantz) storage roots [J]. Plant Molecular Biology, 2017, 94: 185−195. doi: 10.1007/s11103-017-0602-z [19] 张鹏, 杨俊, 周文智, 等. 能源木薯高淀粉抗逆分子育种研究进展与展望 [J]. 生命科学, 2014, 26(5):465−473.ZHANG P, YANG J, ZHOU W Z, et al. Progress and perspective of cassava molecular breeding for bioenergy development [J]. Chinese Bulletin of Life Sciences, 2014, 26(5): 465−473.(in Chinese) [20] HU W, KONG H, GUO Y L, et al. Comparative physiological and transcriptomic analyses reveal the actions of melatonin in the delay of postharvest physiological deterioration of cassava [J]. Frontiers in Plant Science, 2016, 7: 736. [21] XU J, DUAN X G, YANG J, et al. Enhanced reactive oxygen species scavenging by overproduction of superoxide dismutase and catalase delays postharvest physiological deterioration of cassava storage roots [J]. Plant Physiology, 2013, 161(3): 1517−1528. doi: 10.1104/pp.112.212803 [22] ZIDENGA T, LEYVA-GUERRERO E, MOON H, et al. Extending cassava root shelf life via reduction of reactive oxygen species production [J]. Plant Physiology, 2012, 159(4): 1396−1407. doi: 10.1104/pp.112.200345 [23] 张振文, 李开绵. 木薯块根采后腐烂及贮藏方法研究进展 [J]. 热带作物学报, 2012, 33(7):1326−1331. doi: 10.3969/j.issn.1000-2561.2012.07.035ZHANG Z W, LI K M. Review on postharvest deterioration and methods of storage for cassava tuberous root [J]. Chinese Journal of Tropical Crops, 2012, 33(7): 1326−1331.(in Chinese) doi: 10.3969/j.issn.1000-2561.2012.07.035 [24] 曾坚, 廖凤凤, 吴春来, 等. 木薯MeHSF7基因克隆及表达分析 [J]. 南方农业学报, 2020, 51(6):1256−1264.ZENG J, LIAO F F, WU C L, et al. Cloning and expression analysis of MeHSF7 gene in cassava [J]. Journal of Southern Agriculture, 2020, 51(6): 1256−1264.(in Chinese) [25] 杨方威, 段懿菲, 冯叙桥. 脱落酸的生物合成及对水果成熟的调控研究进展 [J]. 食品科学, 2016, 37(3):266−272. doi: 10.7506/spkx1002-6630-201603046YANG F W, DUAN Y F, FENG X Q. Advances in biosynthesis of abscisic acid and its roles in regulation of fruit ripening [J]. Food Science, 2016, 37(3): 266−272.(in Chinese) doi: 10.7506/spkx1002-6630-201603046 [26] MEGA R, ABE F, KIM J S, et al. Tuning water-use efficiency and drought tolerance in wheat using abscisic acid receptors [J]. Nature Plants, 2019, 5(2): 153−159. doi: 10.1038/s41477-019-0361-8 [27] VANDERSCHUREN H, NYABOGA E, POON J S, et al. Large-scale proteomics of the cassava storage root and identification of a target gene to reduce postharvest deterioration [J]. Plant Cell, 2014, 26(5): 1913−1924. doi: 10.1105/tpc.114.123927 [28] OWITI, J, GROSSMANN, J, GEHRIG, P, et al. iTRAQ-based analysis of changes in the cassava root proteome reveals pathways associated with post-harvest physiological deterioration [J]. Plant Journal, 2011, 67(1): 145−156. doi: 10.1111/j.1365-313X.2011.04582.x -







 下载:
下载: