Transcriptome Analysis on Tubers of Two Huaiyushan Cultivated Varieties of Tetrastigma hemsleyanum
-
摘要:
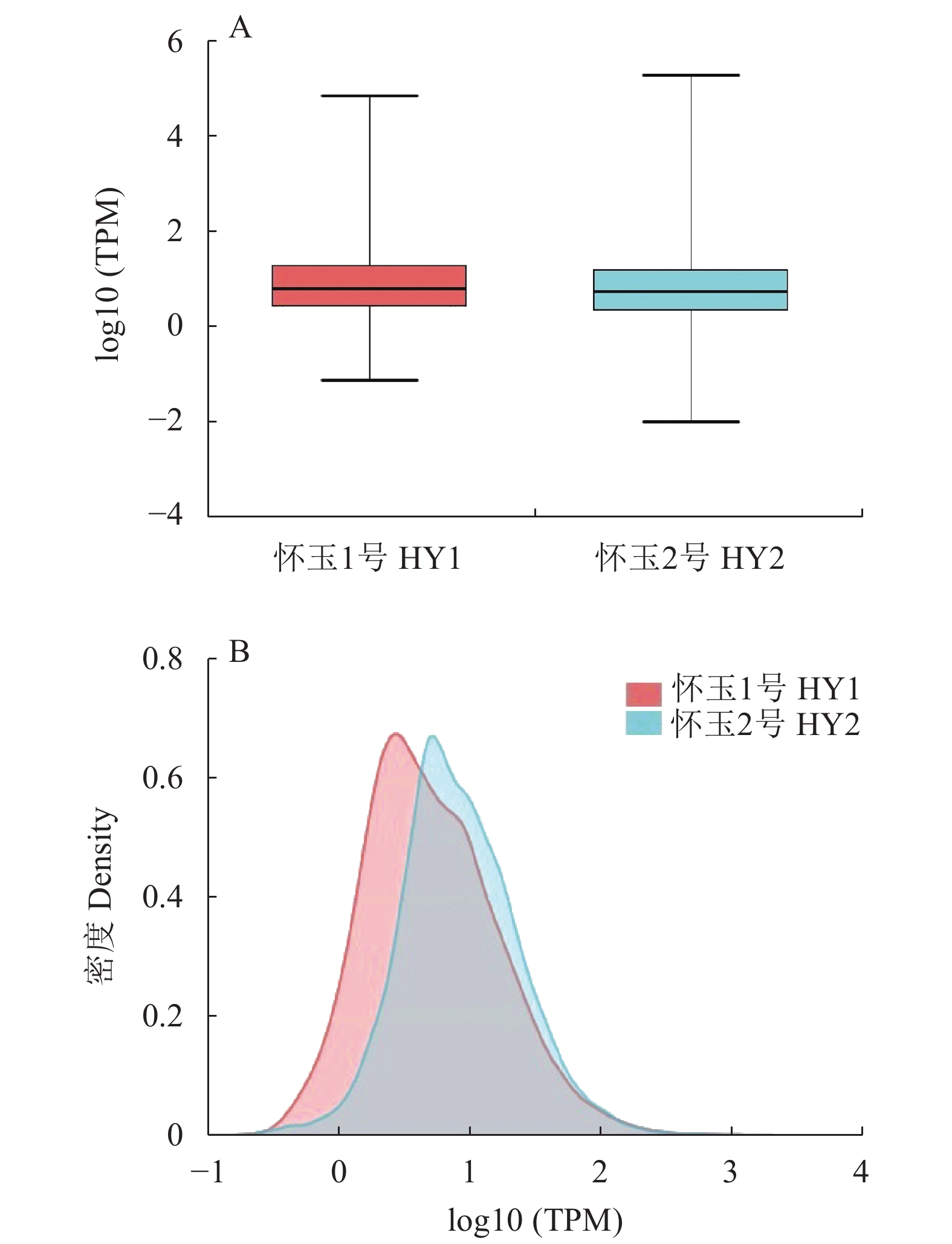
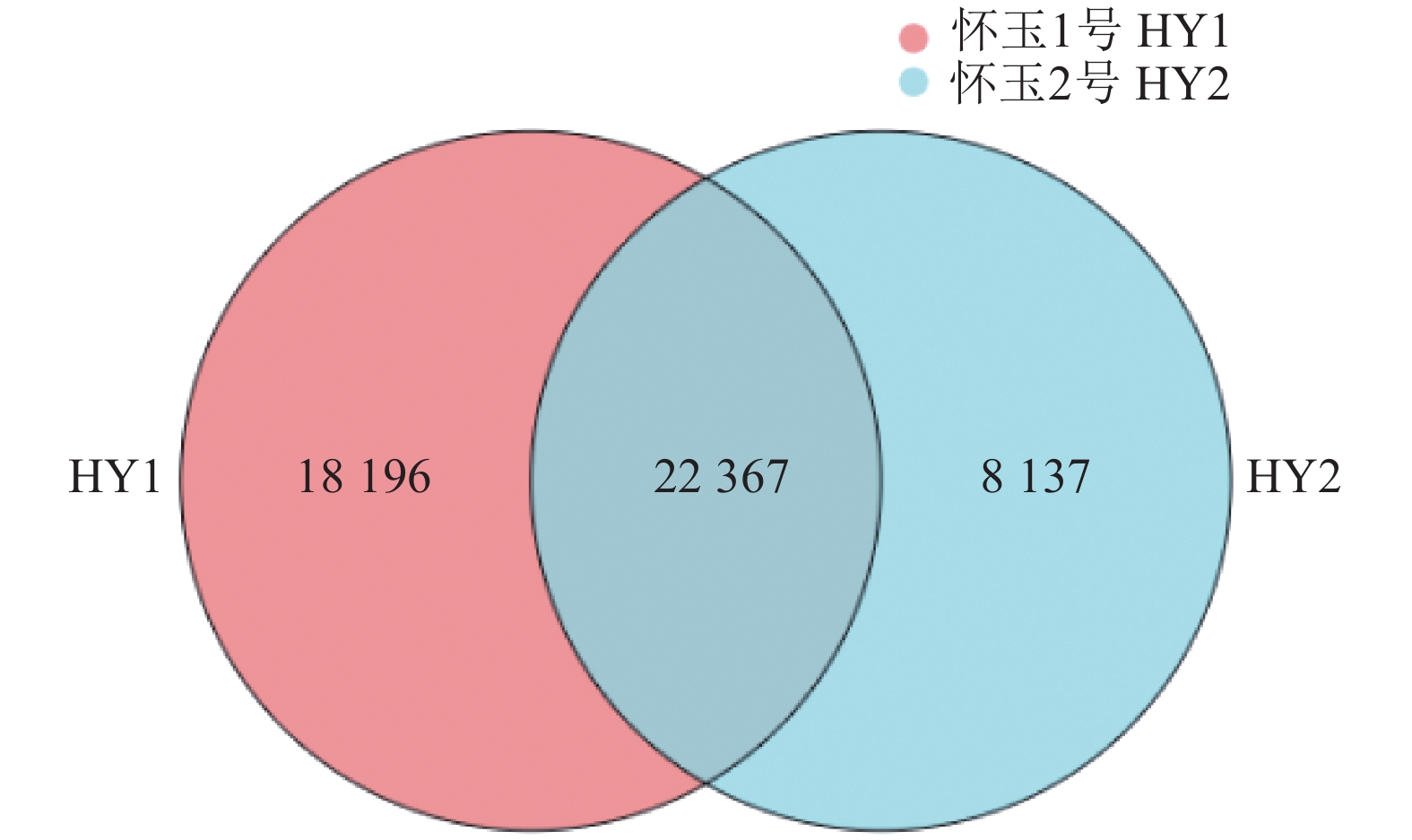
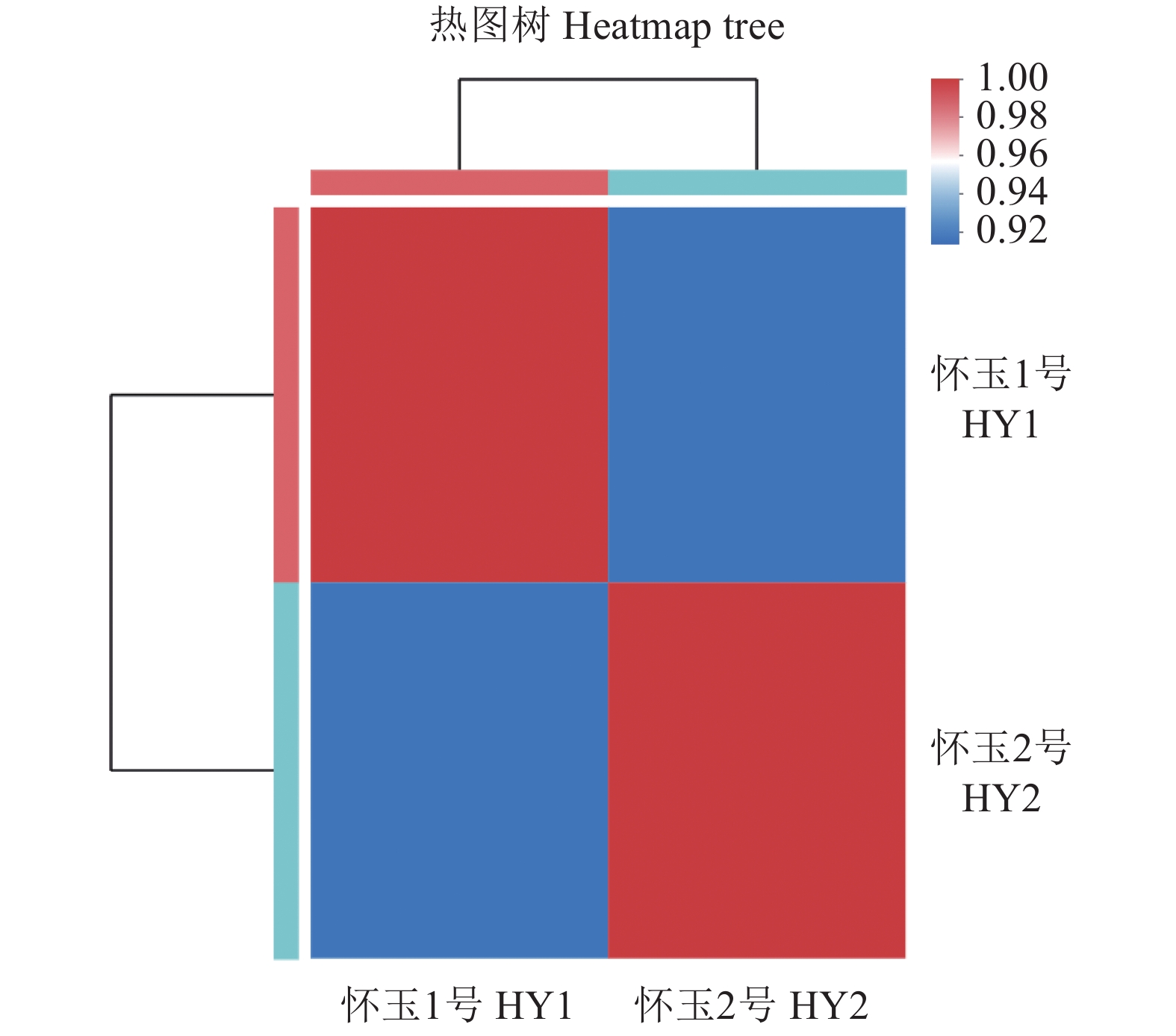
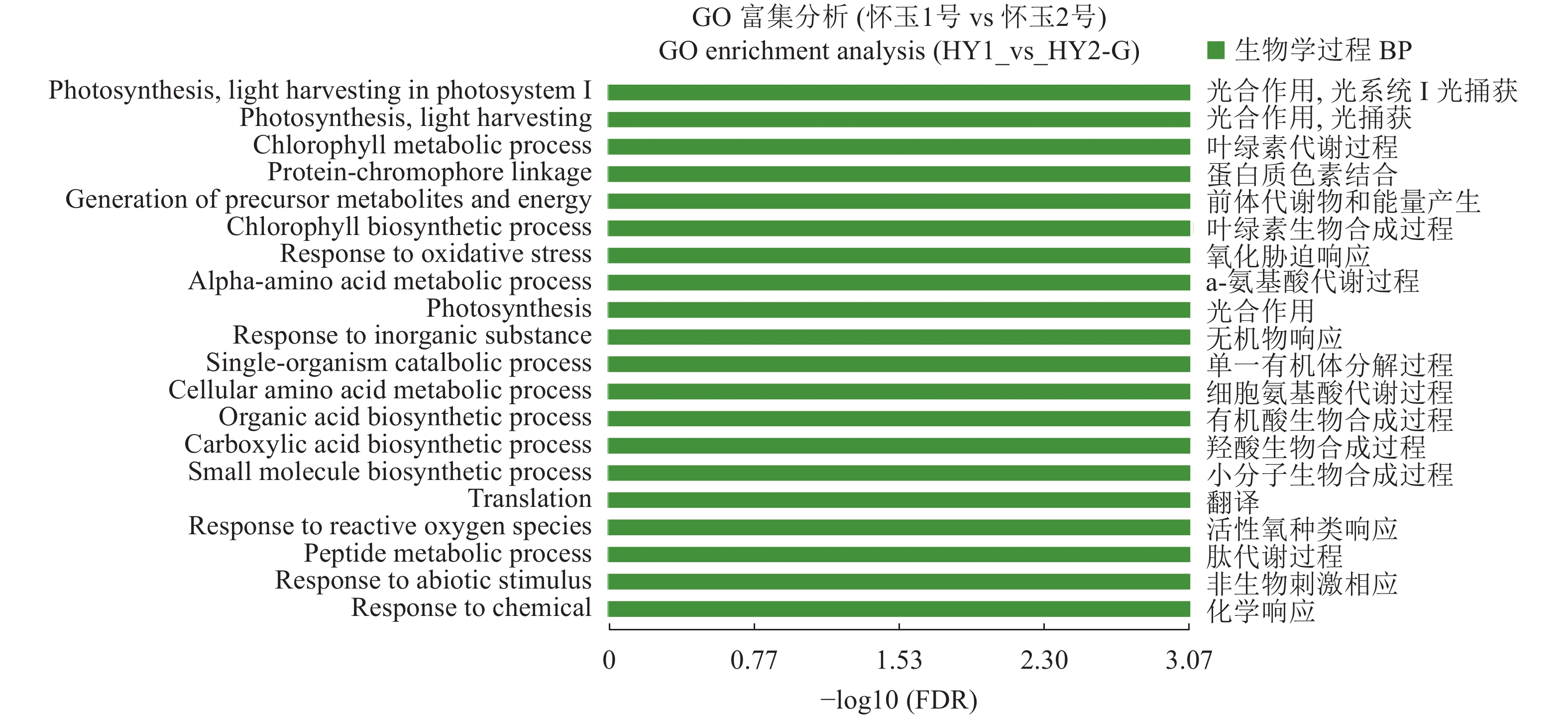
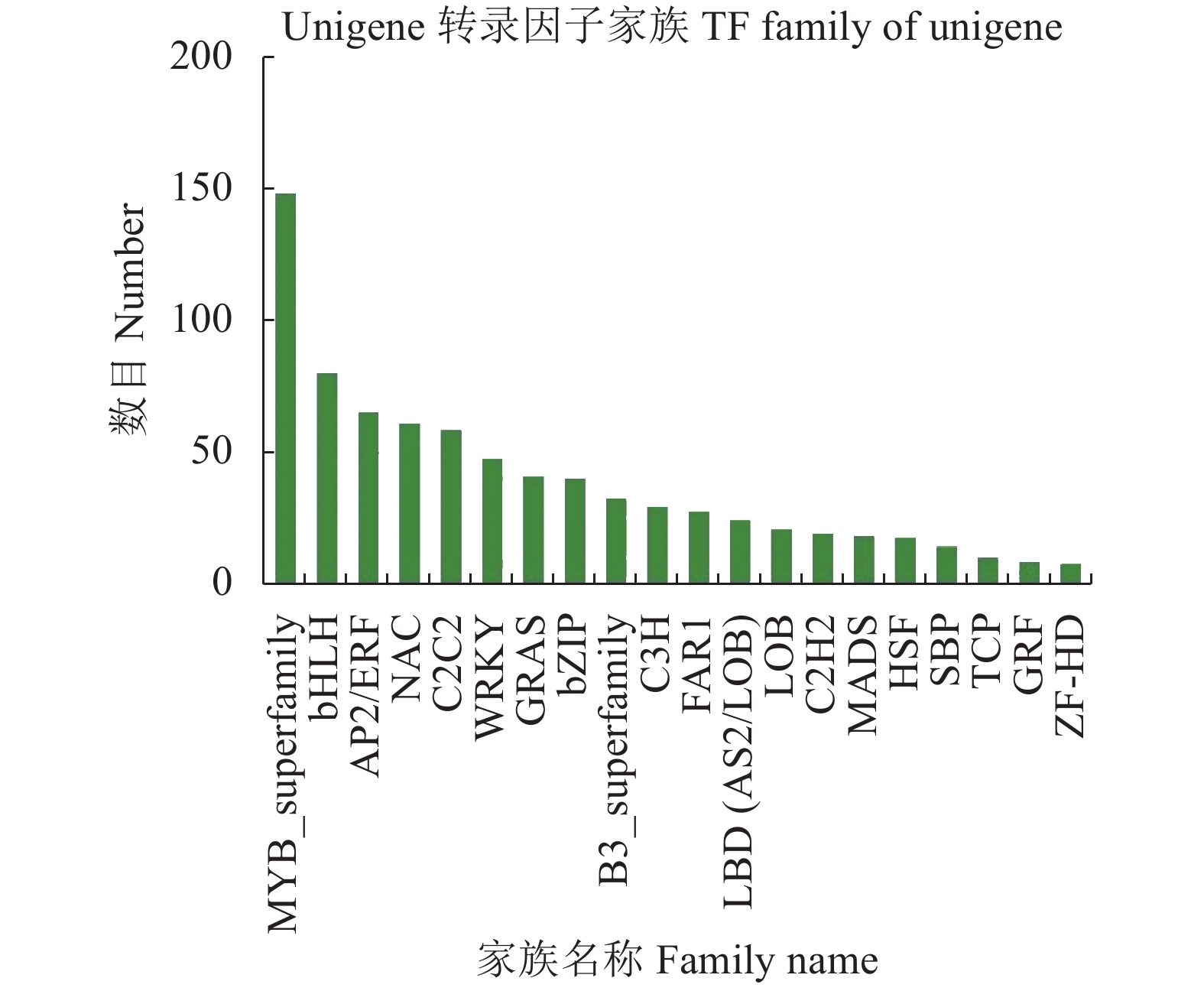
目的 筛选怀玉山三叶青2个栽培种:怀玉1号(HY1组)和怀玉2号(HY2组)与黄酮类化合物合成相关差异表达基因。 方法 以怀玉山三叶青怀玉1号(HY1组)和怀玉2号(HY2组)的块根为试验材料进行转录组分析。 结果 HY1组和HY2组的Clean reads分别为42311662和41411202。2组样品Q30碱基百分比均不小于95.75%。HY1组和HY2组的转录因子家族多为MYB-superfamily、bHLH、AP2/ERF、NAC、C2C2、WRKY等。HY1组和HY2组表达量FPKM的对数值在0-2。HY1组和HY2组表达量密度在0-0.7。HY1组和HY2组表达的共有基因数为22367,HY1组单独表达的基因数为18196,HY2组单独表达的基因数为8137。HY1组和HY2组表达量的相关系数为0.913,样本间相关性好。HY1组和HY2组共产生差异表达基因12199 个。与HY1组比较,HY2组上调基因数为3551,下调基因数为8648。GO富集分析显示,差异基因主要注释到光合作用光系统I光捕获、光合作用捕获、叶绿素代谢过程、蛋白质发色团连锁、前体代谢产物和能量的产生、叶绿素生物合成过程、氧化应激反应、α-氨基酸代谢过程、光合作用、质体小叶、光系统I、光系统II、质体类核仁、光系统、叶绿素结合、单加氧酶活性、铁离子结合、血红素结合、裂解酶活性功能。KEGG富集分析显示,差异基因主要注释到光合作用-天线蛋白、核糖体、乙醛酸和二元酸代谢、苯丙酸生物合成、二苯乙烯类、二芳基庚烷类和姜辣素的生物合成、类黄酮生物合成、光合作用、光合生物的固碳作用、甘氨酸、丝氨酸和苏氨酸代谢、植物激素信号转导、谷胱甘肽代谢、丙酮酸代谢、苯丙氨酸代谢、植物昼夜节律、黄酮和黄酮醇的生物合成、半胱氨酸与蛋氨酸代谢、氰胺酸代谢、类胡萝卜素生物合成、α-亚麻酸代谢、卟啉与叶绿素代谢等代谢途径。 结论 与黄酮类化合物相关差异表达基因如芪合酶(stilbene synthase)、无色花色素双加氧酶(leucoanthocyanidin dioxygenase)、查尔酮异构酶蛋白(CHI protein)、查尔酮合酶2(chalcone synthase 2)、黄烷酮3-羟化酶(flavanone 3-hydroxylase)、无色花色素还原酶(1leucoanthocyanidin reductase 1)、类黄酮3'-羟化酶(flavonoid 3'-hydroxylase)基因在怀玉2号(HY2组)块根中上调,而查尔酮合酶(chalcone synthase)、黄酮醇合酶(flavonol synthase)、类黄酮3',5'-甲基转移酶(flavonoid 3', 5'-methyltransferase)基因在怀玉2号(HY2组)块根中下调,导致怀玉山三叶青怀玉1号(HY1组)和怀玉2号(HY2组)块根总黄酮含量的差异。 Abstract:Objective Transcriptomes of differentially expressed genes related to flavonoids synthesis in tubers of two Huaiyushan cultivated varieties of Tetrastigma hemsleyanum Diels et Gilg were compared. Method Tubers from Huaiyu 1 (HY1) and Huaiyu 2 (HY2) were used for the transcriptome analysis. Result HY1 and HY2 had clean reads of 42 311 662 and 41 411 202, respectively, and no less than 95.75% of Q30 base. Their transcription factors basically belonged to the MYB-superfamily, bHLH, AP2/ERF, NAC, C2C2, WRKY, etc. The paired values of FPKM in HY1 and HY2 were between 0 and 2; the expression densities, between 0 and 0.7; the number of commonly expressed genes, 22 367; and, the number of uniquely expressed genes in HY1, 18 196, while 8 137 in HY2. The correlation between the expressions of the two had a coefficient of 0.913, and that between the samples was high. There were 12 199 differentially expressed genes between the two, with 3 551 upregulated and 8 648 downregulated in HY2 as compared to HY1. The GO enrichment analysis showed that the differential genes were mainly annotated into photosynthesis, light harvesting in photosystem I, photosynthesis, light harvesting, chlorophyll metabolic process, protein-chromophore linkage, generation of precursor metabolites and energy, chlorophyll biosynthetic process, response to oxidative stress, alpha-amino acid metabolic process, photosynthesis, plastoglobule, photosystem I, photosystem II, plastid nucleoid, photosystem, chlorophyll binding, monooxygenase activity, iron ion binding, heme binding, lyase activity, etc. Whereas, the KEGG enrichment analysis indicated the differential genes to be mainly annotated into photosynthesis-antenna proteins, ribosome, glyoxylate and dicarboxylate metabolism, phenylpropanoid biosynthesis, stilbenoid, diarylheptanoid and gingerol biosynthesis, flavonoid biosynthesis, photosynthesis, carbon fixation in photosynthetic organisms, glycine, serine and threonine metabolism, plant hormone signal transduction, glutathione metabolism, pyruvate metabolism, phenylalanine metabolism, circadian rhythm-plant, flavone and flavonol biosynthesis, cysteine and methionine metabolism, cyanoamino acid metabolism, carotenoid biosynthesis, alpha-linolenic acid metabolism, porphyrin and chlorophyll metabolism, and other metabolic pathways. Conclusion The differentially expressed genes related to flavonoids synthesis, such as stilbene synthase, leucoanthocyanidin dioxygenase, CHI protein, chalcone synthase 2, flavanone 3-hydroxylase and lleucoanthocyanidin reductase 1 and flavonoid 3’- hydroxylase gene were upregulated in HY2, while chalcone synthase, flavonol synthase and flavonoid 3’, 5’-methyltransferase downregulated. The variations apparently resulted in the differences shown on the total flavonoid content between the HY1 and HY2 tubers. -
表 1 HY1组和HY2组前15个表达量差异基因(HY1 vs HY2)
Table 1. Top 15 genes in HY1 and HY2 with greatest differences on gene expression (HY1 vs. HY2)
序列编号
Seq_id长度
LengthP值
P value校正 P值
P adjust显著性
Significant调节
Regulate基因名称
Gene nameTRINITY_DN2864_c0_g1 4161 6.01E-34 3.05E-29 是 yes 下调 down 未注释 No TRINITY_DN10874_c0_g1 1930 3.00E-27 7.61E-23 是 yes 上调 up 类核糖体失活蛋白
SNAIfribosome-inactivating protein SNAIf-likeTRINITY_DN4409_c0_g1 721 2.32E-26 2.94E-22 是 yes 下调 down LHCII类1型叶绿素 a-b结合蛋白
chlorophyll a-b binding protein of LHCII type 1-likeTRINITY_DN1538_c0_g1 562 3.20E-26 3.25E-22 是 yes 上调 up 未注释 No TRINITY_DN6900_c0_g1 1888 2.26E-26 3.82E-22 是 yes 下调 down 叶绿体核酮糖二磷酸羧化酶/加氧酶激活酶
ARibulose bisphosphate carboxylase/oxygenase activase A, chloroplasticTRINITY_DN2027_c0_g1 1216 1.68E-25 1.42E-21 是 yes 下调 down 叶绿体产氧增强蛋白1
oxygen-evolving enhancer protein 1, chloroplasticTRINITY_DN151_c0_g1 1068 3.65E-25 2.64E-21 是 yes 下调 down 核酮糖-1,5-二磷酸羧化酶/加氧酶小亚基
ribulose-1,5-bisphosphate carboxylase/oxygenase small subunitTRINITY_DN119_c0_g2 1489 8.14E-25 5.16E-21 是 yes 下调 down 叶绿体甘油醛-3-磷酸脱氢酶
Aglyceraldehyde-3-phosphate dehydrogenase A, chloroplasticTRINITY_DN365_c0_g1 2576 1.08E-24 6.11E-21 是 yes 下调 down 叶绿体转酮酶
transketolase, chloroplasticTRINITY_DN178_c0_g1 896 3.20E-24 1.48E-20 是 yes 下调 down 部分预测蛋白
predicted protein, partialTRINITY_DN640_c0_g1 1411 3.11E-24 1.58E-20 是 yes 下调 down 叶绿体硫胺素噻唑合酶2
thiamine thiazole synthase 2, chloroplasticTRINITY_DN5576_c0_g1 7575 3.75E-24 1.58E-20 是 yes 下调 down 转座子 Tf-2多蛋白
Transposon Tf2-2 polyprotein [Vitis vinifera]TRINITY_DN757_c0_g1 1330 4.58E-24 1.79E-20 是 yes 下调 down 假定碳酸酐酶
putative carbonic anhydraseTRINITY_DN75_c0_g2 1595 8.89E-24 3.22E-20 是 yes 下调 down 果糖-1,6-二磷酸醛缩酶8
fructose-1,6-bisphosphate aldolase 8TRINITY_DN922_c0_g1 1199 9.98E-24 3.38E-20 是 yes 下调 down LHCIIⅢ型叶绿素 a-b结合蛋白
chlorophyll a-b binding protein of LHCII type III, chloroplastic表 2 HY1组和HY2组部分差异表达基因GO富集结果
Table 2. GO enrichment on certain differentially expressed genes in HY1 and HY2
富集到该 GO term的
基因/转录本数目
Number of genes enriched
to the GO termGO Term对
应的编号
ID corresponding
to go termGO三大分类
Three categories
of GOGO功能描述
Go function description该 GO在目标
基因集中
占有的
比例
Proportion of
the GO
in the target
gene set/%该 GO在背景
基因/转录
本中占有
的比例
Proportion of
the go
in the
background gene/%矫正后
的P值
P value_
corrected46 GO:0009768 生物学过程 BP 光合作用光系统 I光捕获
photosynthesis, light harvesting in photosystem I0.42 0.18 0.0009 57 GO:0009765 生物学过程 BP 光合作用光捕获
photosynthesis, light harvesting0.52 0.23 0.0009 44 GO:0015994 生物学过程 BP 叶绿素代谢过程
chlorophyll metabolic process0.40 0.21 0.0009 65 GO:0018298 生物学过程 BP 蛋白质发色团连锁
protein-chromophore linkage0.59 0.33 0.0009 213 GO:0006091 生物学过程 BP 前体代谢产物和能量的产生
generation of precursor metabolites and energy1.95 1.35 0.0009 31 GO:0015995 生物学过程 BP 叶绿素生物合成过程
chlorophyll biosynthetic process0.28 0.14 0.0009 139 GO:0006979 生物学过程 BP 氧化应激反应
response to oxidative stress1.27 0.90 0.0009 227 GO:1901605 生物学过程 BP α-氨基酸代谢过程
alpha-amino acid metabolic process2.08 1.57 0.0009 87 GO:0015979 生物学过程 BP 光合作用
photosynthesis0.80 0.52 0.0009 69 GO:0010287 细胞组分 CC 质体小叶
plastoglobule0.63 0.29 0.0009 65 GO:0009522 细胞组分 CC 光系统 I
photosystem I0.59 0.31 0.0009 68 GO:0009523 细胞组分 CC 光系统 II
photosystem II0.62 0.30 0.0009 36 GO:0042646 细胞组分 CC 质体类核仁
plastid nucleoid0.33 0.17 0.0009 78 GO:0009521 细胞组分 CC 光系统
photosystem0.71 0.39 0.0009 61 GO:0016168 分子功能 MF 叶绿素结合
chlorophyll binding0.56 0.29 0.0009 188 GO:0004497 分子功能 MF 单加氧酶活性
monooxygenase activity1.72 1.15 0.0009 208 GO:0005506 分子功能 MF 铁离子结合
iron ion binding1.90 1.39 0.0009 228 GO:0020037 分子功能 MF 血红素结合
heme binding2.08 1.53 0.0009 276 GO:0016829 分子功能 MF 裂解酶活性
lyase activity2.52 1.91 0.0009 表 3 HY1组和HY2组差异表达基因KEGG富集分析(前20)
Table 3. KEGG enrichment on top 20 differentially expressed genes in HY1 and HY2
数量
Number途径编号
Pathway ID描述
DescriptionKEGG在目标
基因集中
占有的比例
Ratio in study/%KEGG在背景
中占有
的比例
Ratio in pop/%校正后
P值
P value
corrected57 map00196 光合作用-天线蛋白
Photosynthesis-antenna proteins1.00 0.46 0.0000 306 map03010 核糖体
Ribosome5.41 4.42 0.0002 90 map00630 乙醛酸和二元酸代谢
Glyoxylate and dicarboxylate metabolism1.59 1.08 0.0003 100 map00940 苯丙酸生物合成
Phenylpropanoid biosynthesis1.77 1.24 0.0003 31 map00945 二苯乙烯类、二芳基庚烷类和姜辣素的生物合成
Stilbenoid, diarylheptanoid and gingerol biosynthesis0.55 0.29 0.0003 34 map00941 类黄酮生物合成
Flavonoid biosynthesis0.60 0.35 0.0018 64 map00195 光合作用
Photosynthesis1.13 0.77 0.0019 82 map00710 光合生物的固碳作用
Carbon fixation in photosynthetic organisms1.45 1.04 0.0024 71 map00260 甘氨酸、丝氨酸和苏氨酸代谢
Glycine, serine and threonine metabolism1.26 0.89 0.0030 132 map04075 植物激素信号转导
Plant hormone signal transduction2.34 1.85 0.0075 77 map00480 谷胱甘肽代谢
Glutathione metabolism1.36 1.02 0.0119 92 map00620 丙酮酸代谢
Pyruvate metabolism1.63 1.25 0.0120 41 map00360 苯丙氨酸代谢
Phenylalanine metabolism0.73 0.49 0.0165 50 map04712 植物昼夜节律
Circadian rhythm - plant0.88 0.63 0.0187 6 map00944 黄酮和黄酮醇的生物合成
Flavone and flavonol biosynthesis0.11 0.04 0.0217 89 map00270 半胱氨酸与蛋氨酸代谢
Cysteine and methionine metabolism1.57 1.23 0.0221 42 map00460 氰胺酸代谢
Cyanoamino acid metabolism0.74 0.52 0.0222 36 map00906 类胡萝卜素生物合成
Carotenoid biosynthesis0.64 0.44 0.0343 44 map00592 α-亚麻酸代谢
alpha-Linolenic acid metabolism0.78 0.56 0.0398 46 map00860 卟啉与叶绿素代谢
Porphyrin and chlorophyll metabolism0.81 0.60 0.0445 表 4 与次生代谢物合成相关差异基因的表达
Table 4. Expressions of differentially expressed genes related to secondary metabolites synthesis
基因ID
Gene id基因名称
Gene nameHY1 HY2 调节
RegulateTRINITY_DN10561_c0_g1 芪合酶 stilbene synthase 4.64 23.15 上调 up TRINITY_DN12750_c0_g1 无色花色素双加氧酶 leucoanthocyanidin dioxygenase 14.07 50.17 上调 up TRINITY_DN1644_c0_g1 查尔酮异构酶蛋白 CHI protein 82.09 160.88 上调 up TRINITY_DN16981_c0_g4 查尔酮合酶 chalcone synthase 7.15 0 下调 down TRINITY_DN30140_c0_g1 黄酮醇合酶 flavonol synthase 5.44 2.92 下调 down TRINITY_DN43_c0_g1 查尔酮合酶2chalcone synthase 2 32.52 281.84 上调 up TRINITY_DN6146_c0_g1 黄烷酮3-羟化酶 flavanone 3-hydroxylase 54.15 64.75 上调 up TRINITY_DN6563_c0_g1 无色花色素还原酶1 leucoanthocyanidin reductase 1 4.91 25.68 上调 up TRINITY_DN22758_c0_g2 类黄酮3′,5′-甲基转移酶 flavonoid 3′,5′-methyltransferase 7.41 1.15 下调 down TRINITY_DN4109_c0_g1 类黄酮3′-羟化酶 flavonoid 3′-hydroxylase 18.35 82.99 上调 up -
[1] 叶子飘, 谢志亮, 段世华, 等. 设施栽培条件下三叶青叶片光合的气孔和非气孔限制 [J]. 植物生理学报, 2020, 56(1):41−48.YE Z P, XIE Z L, DUAN S H, et al. Stomatal and non-stomatal limitation of photosynthesis for Tetrastigma hemsleyanum under the condition of facility cultivation [J]. Plant Physiology Journal, 2020, 56(1): 41−48.(in Chinese) [2] SUN Y, LI H Y, HU J N, et al. Qualitative and quantitative analysis of phenolics in Tetrastigma hemsleyanum and their antioxidant and antiproliferative activities [J]. Journal of Agricultural and Food Chemistry, 2013, 61(44): 10507−10515. doi: 10.1021/jf4037547 [3] ZHONG L R, ZHENG J X, SUN Q Q, et al. Radix tetrastigma hemsleyani flavone inhibits proliferation, migration, and invasion of human lung carcinoma A549 cells [J]. OncoTargets and Therapy, 2016, 9(1): 635−641. [4] FENG Z Q, HAO W R, LIN X Y, et al. Antitumor activity of total flavonoids from Tetrastigma hemsleyanum Diels et Gilg is associated with the inhibition of regulatory T cells in mice [J]. OncoTargets and Therapy, 2014, 7: 947−956. [5] PENG X, ZHANG Y Y, WANG J, et al. Ethylacetate extract from Tetrastigma hemsleyanum induces apoptosis via the mitochondrial caspase-dependent intrinsic pathway in HepG2 cells [J]. Tumor Biology, 2016, 37(1): 865−876. doi: 10.1007/s13277-015-3579-8 [6] XU C J, DING G Q, FU J Y, et al. Immunoregulatory effects of ethyl-acetate fraction of extracts from Tetrastigma hemsleyanum Diels et. gilg on immune functions of ICR mice [J]. Biomedical and Environmental Sciences, 2008, 21(4): 325−331. doi: 10.1016/S0895-3988(08)60050-1 [7] 李士敏, 李强, 孙崇鲁, 等. 基于多模式识别结合指纹图谱的三叶青产地鉴别比较研究 [J]. 中草药, 2020, 51(1):197−203. doi: 10.7501/j.issn.0253-2670.2020.01.026LI S M, LI Q, SUN C L, et al. Comparative study on multiple chemical pattern recognition combined with fingerprint ofTetrastigma hemsleyanum from different habitats [J]. Chinese Traditional and Herbal Drugs, 2020, 51(1): 197−203.(in Chinese) doi: 10.7501/j.issn.0253-2670.2020.01.026 [8] SUN Y, HUI Q R, CHEN R, et al. Apoptosis in human hepatoma HepG2 cells induced by the phenolics of Tetrastigma hemsleyanum leaves and their antitumor effects in H22 tumor-bearing mice [J]. Journal of Functional Foods, 2018, 40: 349−364. doi: 10.1016/j.jff.2017.11.017 [9] SUN Y, QIN Y, LI H Y, et al. Rapid characterization of chemical constituents in Radix Tetrastigma, a functional herbal mixture, before and after metabolism and their antioxidant/antiproliferative activities [J]. Journal of Functional Foods, 2015, 18: 300−318. doi: 10.1016/j.jff.2015.07.009 [10] SUN Y, TSAO R, CHEN F, et al. The phytochemical composition, metabolites, bioavailability and in vivo antioxidant activity of Tetrastigma hemsleyanum leaves in rats [J]. Journal of Functional Foods, 2017, 30: 179−193. doi: 10.1016/j.jff.2017.01.004 [11] SUN Y, TSAO R, CHEN F, et al. The phenolic profiles of Radix tetrastigma after solid phase extraction (SPE) and their antitumor effects and antioxidant activities in H22 tumor-bearing mice [J]. Food & Function, 2017, 8(11): 4014−4027. [12] LU T, LU G, FAN D, et al. Function annotation of the rice transcriptome at single-nucleotide resolution by RNA-seq [J]. Genome Research, 2010, 20(9): 1238−1249. doi: 10.1101/gr.106120.110 [13] WANG Z Y, FANG B P, CHEN J Y, et al. De novo assembly and characterization of root transcriptome using Illumina paired-end sequencing and development of cSSR markers in sweetpotato (Ipomoea batatas) [J]. BMC Genomics, 2010, 14: 125−135. [14] ZHANG J N, LIANG S, DUAN J L, et al. De novo assembly and Characterisation of the Transcriptome during seed development, and generation of genic-SSR markers in Peanut (Arachis hypogaea L.) [J]. BMC Genomics, 2012, 13(1): 90−95. doi: 10.1186/1471-2164-13-90 [15] 魏俊雯, 张声祥, 施圆圆, 等. 基于转录组测序的牛蒡木质素类物质生物合成途径及关键酶基因分析 [J]. 中草药, 2020, 51(16):4300−4307. doi: 10.7501/j.issn.0253-2670.2020.16.026WEI J W, ZHANG S X; SHI Y Y. Transcriptome analysis reveals key enzyme genes involved in lignin biosynthesis pathway in Arctium lappa [J]. Chinese Traditional and Herbal Drugs, 2020, 51(16): 4300−4307.(in Chinese) doi: 10.7501/j.issn.0253-2670.2020.16.026 [16] 孙健, 沈晓霞, 陈加红, 等. 药用植物三叶青种质多样性与栽培管理的研究进展 [J]. 科技通报, 2018, 34(1):13−17.SUN J, SHEN X X, CHEN J H, et al. Germplasm diversity and cultural management of the medicinal plant Tetrastigma hemsleyanum [J]. Bulletin of Science and Technology, 2018, 34(1): 13−17.(in Chinese) [17] 林国卫, 闻静, 石光禹, 等. 侵染怀玉山三叶青的病毒 RT-PCR 鉴定 [J]. 分子植物育种, 2020, 18(3):968−975.LIN G W, WEN J, SHI G Y, et al. Identification of Viruses Infecting Huaiyushan Tetrastigma hemsleyanum Diels et Gilg by RT-PCR [J]. Molecular Plant Breeding, 2020, 18(3): 968−975.(in Chinese) [18] 张雪松, 裴建军, 赵林果, 等. 不同品种桂花转录组分析及桂花精油成分差异的初步探讨 [J]. 天然产物研究与开发, 2016, 28(4):529−535.ZHANG X S, PEI J J, ZHAO L G, et al. Transcriptome analysis of different Osmanthus reveals insight into the difference of Osmanthus oil components [J]. Natural Product Research and Development, 2016, 28(4): 529−535.(in Chinese) [19] 张驰, 高振蕊, 董友魁, 等. 四个大豆栽培种的花序转录组分析 [J]. 生态学杂志, 2015, 34(12):3391−3396.ZHANG C, GAO Z R, DONG Y K, et al. Transcriptome analysis of inflorescences from four soybean cultivars [J]. Chinese Journal of Ecology, 2015, 34(12): 3391−3396.(in Chinese) [20] 成启明, 格根图, 撒多文, 等. 不同品种紫花苜蓿转录组分析及营养品质差异的探讨 [J]. 草业学报, 2019, 28(10):199−208. doi: 10.11686/cyxb2018721CHENG Q M, GE G T, SA D W, et al. Transcriptome analyses provide insights into differences in nutritional quality in different alfalfa varieties [J]. Acta Prataculturae Sinica, 2019, 28(10): 199−208.(in Chinese) doi: 10.11686/cyxb2018721 [21] 王宇, 陈 楠, 袁启凤, 等. 3个不同品种百香果转录组分析 [J]. 种子, 2019, 38(5):1−7.WANG Y, CHEN N, YUAN Q F, et al. Transcriptome analysis of three different varieties of passion fruit [J]. Seed, 2019, 38(5): 1−7.(in Chinese) [22] ZHANG J, SUBRAMANIAN S, STACEY G, et al. Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti [J]. The Plant Journal, 2009, 57(1): 171−183. doi: 10.1111/j.1365-313X.2008.03676.x [23] TREUTTER D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis [J]. Plant Biology, 2005, 7(6): 581−591. doi: 10.1055/s-2005-873009 [24] FOWLER Z L, KOFFAS M A G. Biosynthesis and biotechnological production of flavanones: current state and perspectives [J]. Applied Microbiology and Biotechnology, 2009, 83(5): 799−808. doi: 10.1007/s00253-009-2039-z [25] HAIN R, REIF H J, KRAUSE E, et al. Disease resistance results from foreign phytoalexin expression in a novel plant [J]. Nature, 1993, 361(6408): 153−156. doi: 10.1038/361153a0 [26] POULSEN M M, FJELDBORG K, ORNSTRUP M J, et al. Resveratrol and inflammation: Challenges in translating pre-clinical findings to improved patient outcomes [J]. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 2015, 1852(6): 1124−1136. doi: 10.1016/j.bbadis.2014.12.024 [27] HOLME A L, PERVAIZ S. Resveratrol in cell fate decisions [J]. Journal of Bioenergetics and Biomembranes, 2007, 39(1): 59−63. doi: 10.1007/s10863-006-9053-y [28] AHUJA I, KISSEN R, BONES A M. Phytoalexins in defense against pathogens [J]. Trends in Plant Science, 2012, 17(2): 73−90. doi: 10.1016/j.tplants.2011.11.002 [29] CHONG J L, POUTARAUD A, HUGUENEY P. Metabolism and roles of stilbenes in plants [J]. Plant Science, 2009, 177(3): 143−155. doi: 10.1016/j.plantsci.2009.05.012 [30] 吴凤颖, 刘梦琦, 王跃进, 等. 中国野生毛葡萄芪合酶基因抗白粉病功能分析 [J]. 园艺学报, 2020, 47(2):205−219.WU F Y, LIU M Q, WANG Y J, et al. Function analysis of the stilbene synthase genes VqSTS12 and VqSTS25 of the resistance to powdery mildew in vitis quinquangularis [J]. Acta Horticulturae Sinica, 2020, 47(2): 205−219.(in Chinese) [31] 李娟, 刘海峰, 曹芳芳, 等. 山葡萄无色花色素双加氧酶基因(LDOX)cDNA的克隆与表达 [J]. 西北农业学报, 2016, 25(1):103−108. doi: 10.7606/j.issn.1004-1389.2016.01.014LI J, LIU H F, CAO F F, et al. Cloning and Analysis of Leucoanthocyanidin Dioxygenase(LDOX)inVitis amurensis Rupr [J]. Acta Agriculturae Boreali-Occidentalis Sinica, 2016, 25(1): 103−108.(in Chinese) doi: 10.7606/j.issn.1004-1389.2016.01.014 [32] APPELHAGEN I, JAHNS O, BARTELNIEWOEHNER L, et al. Leucoanthocyanidin Dioxygenase in Arabidopsis thaliana: Characterization of mutant alleles and regulation by MYB-BHLH-TTG1 transcription factor complexes [J]. Gene, 2011, 484(1/2): 61−68. [33] TANNER G J, FRANCKI K T, ABRAHAMS S, et al. Proanthocyanidin biosynthesis in plants [J]. Journal of Biological Chemistry, 2003, 278(34): 31647−31656. doi: 10.1074/jbc.M302783200 [34] HOLTON T A, CORNISH E C. Genetics and biochemistry of anthocyanin biosynthesis [J]. The Plant Cell, 1995: 1071−1083. [35] 蔡建平, 侯和胜. 葡萄查耳酮合酶基因克隆及其进化分析 [J]. 天津农业科学, 2015, 21(1):6−8. doi: 10.3969/j.issn.1006-6500.2015.01.002CAI J P, HOU H S. Cloning and Evolution Analysis of Chalcone Synthase from Vitis vinifera [J]. Tianjin Agricultural Sciences, 2015, 21(1): 6−8.(in Chinese) doi: 10.3969/j.issn.1006-6500.2015.01.002 [36] CHENG H, LI L L, CHENG S Y, et al. Molecular cloning and function assay of a Chalcone isomerase gene (GbCHI) from Ginkgo biloba [J]. Plant Cell Reports, 2011, 30(1): 49−62. doi: 10.1007/s00299-010-0943-4 [37] 尹峰, 龙月红, 冯若宣, 等. 多穗柯黄酮 3-羟化酶基因的克隆与序列分析 [J]. 中草药, 2017, 48(24):5085−5089. doi: 10.7501/j.issn.0253-2670.2017.24.005YIN F, LONG Y H, FENG R X, et al. Cloning of flavanone 3-hydroxylase gene from Lithocarpus polystachyus and its sequence analysis [J]. Chinese Traditional and Herbal Drugs, 2017, 48(24): 5085−5089.(in Chinese) doi: 10.7501/j.issn.0253-2670.2017.24.005 [38] YANG H, AHN J H, IBRAHIM R K, et al. The three-dimensional structure of Arabidopsis thaliana O-methyltransferase predicted by homologybased modelling [J]. Journal of Molecular Graphics & Modelling, 2004, 23(1): 77−87. -






 下载:
下载: