Effects of Vectors on Transient Expression of GFP in CHO Cells
-
摘要:
目的 ExpiCHO细胞瞬时表达能够快速有效地生产重组蛋白,为筛选有效的重组蛋白节省了大量的时间,细胞系的选择、表达载体的选择、转染试剂的选择等是制约ExpiCHO细胞瞬时表达蛋白的重要因素,本研究旨在筛选出在CHO细胞中瞬时表达量较高的载体。 方法 以GFP基因作为目的基因,用不同的真核表达载体构建GFP重组质粒,瞬时转染ExpiCHO-S细胞。转染后4 d观察不同载体表达GFP的荧光数量和强度;转染后8 d对细胞进行裂解,收获裂解上清,用SDS-PAGE对不同载体的表达量进行鉴定。同时用His Trap FF亲和层析柱对裂解上清中的GFP蛋白进行纯化,通过Western Blot比较不同载体GFP蛋白的表达量。 结果 pCDNA3.1-GFP、pCDNA3.4-GFP载体表达的荧光数量最多,pCIneo-GFP载体表达的荧光强度最强,pCMVHA载体表达的荧光数量最少,荧光强度最弱。SDS-PAGE和Western Blot结果均表明pCDNA3.1-GFP、pCDNA3.4-GFP、pCIneo-GFP重组质粒的蛋白表达量高于pCHO-GFP、pCMVHA-GFP重组质粒的蛋白表达量。 结论 筛选出表达量较高的真核表达载体pCDNA3.1、pCDNA3.4、pCIneo,为后续重组蛋白的瞬时表达载体选择提供依据。 Abstract:Objective Appropriate cell line, expression vector, and transfection reagent were selected to enhance the transient expression of GFP recombinant protein for quick and efficient production of the proteins in CHO cells. Methods Targeting GFP, various eukaryotic vectors were employed to carry the gene protein for transfection into ExpiCHO-S cells. Four days after transfection, number and intensity of expression fluorescence on the recombinant plasmids by different vectors were recorded. Four more days later, the cells were lysed, and the lysate supernatant harvested to verify the gene expressions by SDS-PAGE. Meanwhile, GFP recombinant plasmids in the supernatant were purified on a His Trap FF affinity chromatography column to quantify the protein expressions by western blot. Results The recombinant plasmids, pCDNA3.1-GFP and pCDNA3.4-GFP had the expression fluorescence the greatest in number, pCIneo-GFP the highest on intensity, while pCMVHA the fewest in number and the lowest on intensity. SDS-PAGE and western blot showed the expressions of the first 3 recombinant plasmids were higher than those of pCHO-GFP or pCMVHA-GFP. Conclusion The GFP recombinant plasmids constructed with the eukaryotic vectors, pCDNA3.1, pCDNA3.4, and pCIneo, exhibited the greatest expressions and were considered the choice vectors for future studies requiring transient expression. -
Key words:
- GFP /
- CHO cells /
- eukaryotic expression vector
-
图 1 不同载体构建GFP重组质粒双酶切结果
注:M1:DNA Marker;1:pCHO-GFP重组质粒酶切;2:pCDNA3.1-GFP重组质粒酶切;3:pCDNA3.4-GFP重组质粒酶切;4:pCIneo-GFP重组质粒酶切;5:pCMVHA-GFP重组质粒酶切;M2:DNA Marker。
Figure 1. Restriction map of GFP recombinant plasmids carried by different vectors
Note: M1: DNA marker; 1: digestion site for pCHO-GFP recombinant plasmid; 2: digestion site for pCDNA3.1-GFP recombinant plasmid; 3: digestion site for pCDNA3.4-GFP recombinant plasmid; 4: digestion site for pCIneo-GFP recombinant plasmid; 5: digestion site for pCMVHA-GFP recombinant plasmid; M2: DNA marker.
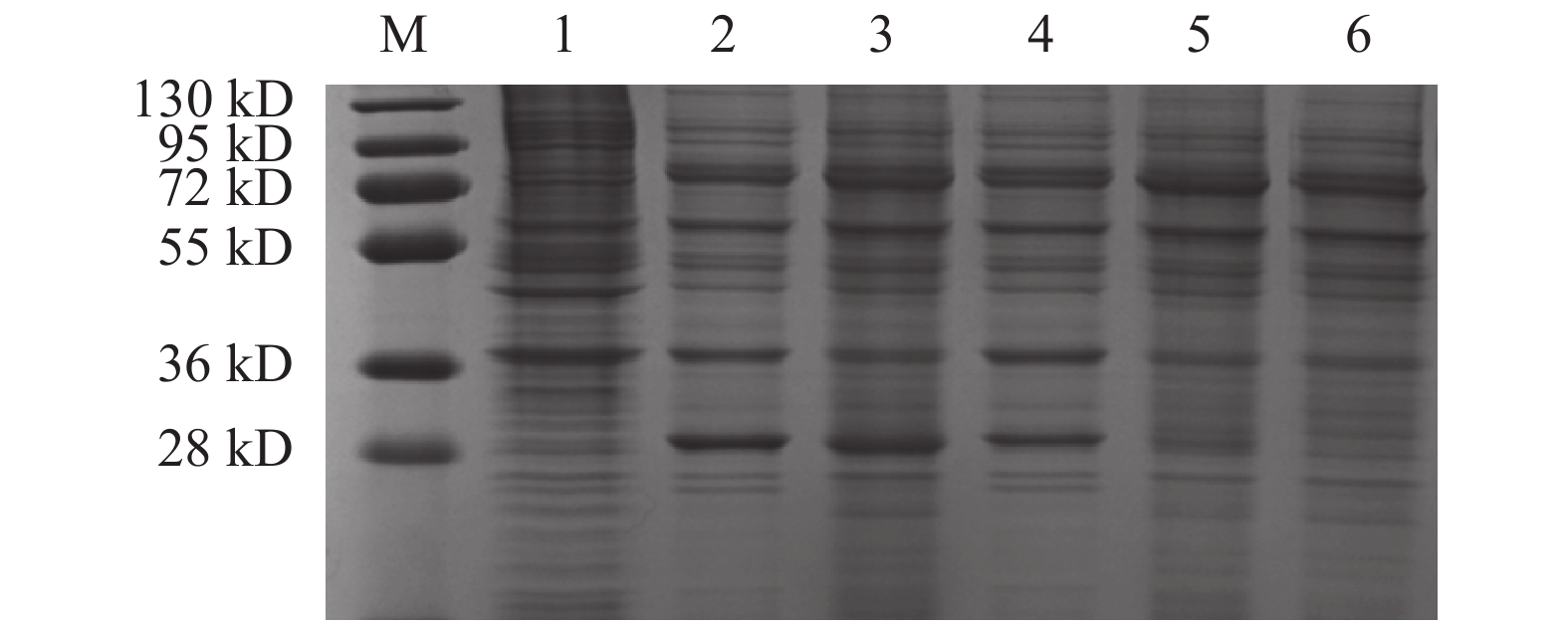
图 3 SDS-PAGE分析转染后4 d GFP蛋白的表达
注:M:Protein Marker;1:ExpiCHO细胞裂解上清;2:pCDNA3.1-GFP细胞裂解上清;3:pCDNA3.4-GFP细胞裂解上清;4:pCIneo-GFP细胞裂解上清;5:pCHO-GFP细胞裂解上清;6:pCMVHA-GFP细胞裂解上清。
Figure 3. SDS-PAGE on GFP expression 4 d after transfection
Note: M: Protein marker; 1: ExpiCHO cell lysate supernatant; 2: cell lysate supernatant (pCDNA3.1-GFP); 3: cell lysate supernatant (pCDNA3.4-GFP); 4: cell lysate supernatant (pCIneo-GFP); 5: cell lysate supernatant (pCHO-GFP); 6: cell lysate supernatant (pCMVHA-GFP).
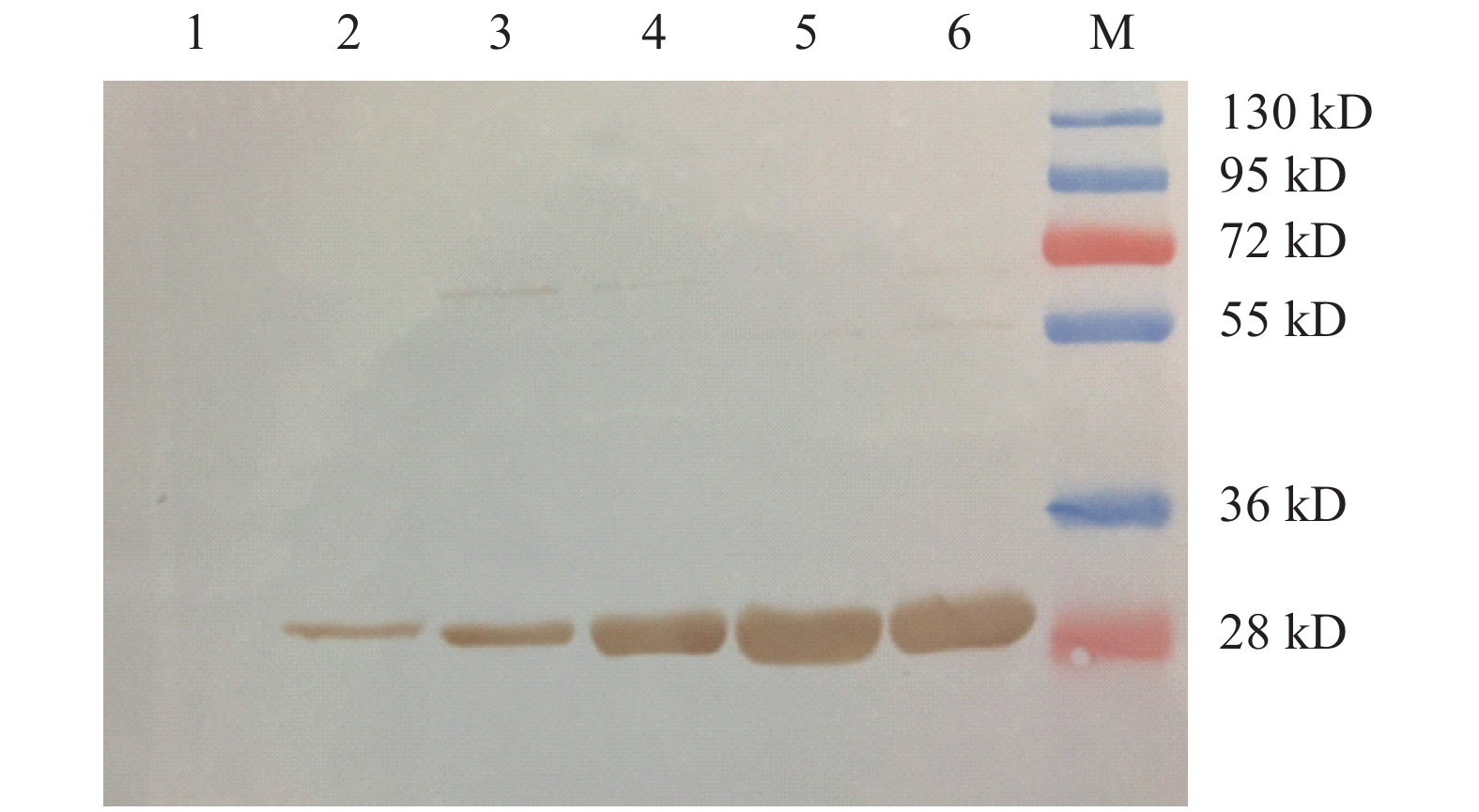
图 4 Western Blot分析纯化后的GFP蛋白
注:1:ExpiCHO细胞裂解上清;2:GFP蛋白(pCMVHA)纯化后;3:GFP蛋白(pCHO)纯化后;4:GFP蛋白(pCIneo)纯化后;5:GFP蛋白(pCDNA3.4)纯化后;6:GFP蛋白(pCDNA3.1)纯化后;M:Protein Marker。
Figure 4. Western blot on purified GFP protein
Note: 1: ExpiCHO cell lysate supernatant; 2: purified GFP protein (pCMVHA); 3: purified GFP protein (pCHO); 4: purified GFP protein (pCIneo); 5: purified GFP protein (pCDNA3.4); 6: purified GFP protein (pCDNA3.1); M: Protein marker.
表 1 不同载体的酶切位点及引物序列
Table 1. Digestion sites and primer sequences on different vectors
载体名
Vectors上游引物序列
Sequence of upstream primers下游引物序列
Sequence of downstream primers上游酶切位点
Upstream restriction site下游酶切位点
Downstream cleavage sitepCDNA3.1 CTAGCTAGCATGGTGAGCAAGGGCGCCGAGCTGTTCACCGGCATC CGGCTCGAGTTAATGATGATGATGATGATGCTTGTACAGCTCATC Nhe I Xho I pCDNA3.4 CTAGTCTAGAATGGTGAGCAAGGGCGCCGAGCTGTTCACCGGCAT GGGAAGCTTTTAATGATGATGATGATGATGCTTGTACAGCTCATC Xba I Hind III pCMVHA CCGGAATTCGCATGGTGAGCAAGGGCGCCGAGCTGTTCACCGGCA CGGCTCGAGTTAATGATGATGATGATGATGCTTGTACAGCTCATC Eco RI Xho I pCIneo CTAGCTAGCATGGTGAGCAAGGGCGCCGAGCTGTTCACCGGCATC CGGCTCGAGTTAATGATGATGATGATGATGCTTGTACAGCTCATC Nhe I Xho I pCHO1.0 CCGCCTAGGGCCACCATGGTGAGCAAGGGCGCCGAGCTGTTCACC CGGGTATACTTAATGATGATGATGATGATGCTTGTACAGCTCATC Avr II Bstz 17I 注:斜体碱基为酶切位点。
Note: The italic bases are restriction sites.表 2 不同载体片段大小
Table 2. Fragment sizes of different vectors
载体名称 Vectors 载体大小 Vectors size/bp pCDNA3.1 5 333 pCDNA3.4 6 010 pCMVHA 3 782 pCIneo 5 747 pCHO 12 988 表 3 不同载体表达GFP蛋白的荧光细胞数
Table 3. Number of fluorescent cells expressing GFP by different vectors
GFP蛋白表达载体
GFP protein
expression vector荧光细胞数量
The number of
fluorescent cellspCDNA3.1 168 pCDNA3.4 110 pCIneo 47 pCHO 151 pCMVHA 58 表 4 不同载体表达GFP蛋白的灰度比值
Table 4. Gray ratio of vectors expressing GFP
GFP蛋白表达载体
GFP protein expression vector灰度比值
Gray ratiopCMVHA 1.00 pCHO 1.36 pCIneo 2.13 pCDNA3.4 2.68 pCDNA3.1 3.19 -
[1] 郭景亮. 中国仓鼠卵巢细胞表达外源蛋白研究进展 [J]. 生物技术世界, 2016(4):321.GUO J L. Research progress of foreign protein expression in Chinese hamster ovary cells [J]. Biotech World, 2016(4): 321.(in Chinese) [2] 温家明, 聂艳峰, 梁翰章, 等. MG-132提高TNFR-Fc融合蛋白在CHO细胞中表达的研究 [J]. 中国生物工程杂志, 2015, 35(9):1−6.WEN J M, NIE Y F, LIANG H Z, et al. MG-132 improve the production of TNFR-Fc fusion protein in CHO cells [J]. China Biotechnology, 2015, 35(9): 1−6.(in Chinese) [3] 韩阳, 蔡洁行, 张朗, 等. 重组蛋白的CHO细胞瞬时表达体系的研究进展 [J]. 药物生物技术, 2017, 24(3):243−248.HAN Y, CAI J H, ZHANG L, et al. Development of CHO system used for transiently expressing recombinant proteins [J]. Pharmaceutical Biotechnology, 2017, 24(3): 243−248.(in Chinese) [4] 孙静静, 李桂林, 周雷鸣, 等. 哺乳动物细胞瞬时转染技术研究进展 [J]. 中国医药生物技术, 2019, 14(3):253−257. doi: 10.3969/j.issn.1673-713X.2019.03.010SUN J J, LI G L, ZHOU L M, et al. Advances in transient transfection technology of mammalian cells [J]. Chinese Medicinal Biotechnology, 2019, 14(3): 253−257.(in Chinese) doi: 10.3969/j.issn.1673-713X.2019.03.010 [5] 刘国奇, 王海涛. 外源蛋白在中国仓鼠卵巢细胞中高效表达的策略 [J]. 生物化学与生物物理进展, 2000, 27(5):496−500. doi: 10.3321/j.issn:1000-3282.2000.05.011G Q, WANG H T. Optimized strategies to hyperexpress recombinant protein in Chinese hamster ovary cells [J]. Progress in Biochemistry and Biophysics, 2000, 27(5): 496−500.(in Chinese) doi: 10.3321/j.issn:1000-3282.2000.05.011 [6] 申烨华, 耿信笃. CHO细胞表达系统研究新进展 [J]. 生物工程进展, 2000, 20(4):23−25, 22. doi: 10.3969/j.issn.1671-8135.2000.04.005SHEN Y H, GENG X D. The recent progress in the upstream studies on the culture with CHO cell [J]. Progress in Biotechnology, 2000, 20(4): 23−25, 22.(in Chinese) doi: 10.3969/j.issn.1671-8135.2000.04.005 [7] OLIVEIRA C, DOMINGUES L. Guidelines to reach high-quality purified recombinant proteins [J]. Applied Microbiology and Biotechnology, 2018, 102(1): 81−92. doi: 10.1007/s00253-017-8623-8 [8] HEINTZMAN N D, REN B. The gateway to transcription: Identifying, characterizing and understanding promoters in the eukaryotic genome [J]. Cellular and Molecular Life Sciences, 2007, 64(4): 386−400. doi: 10.1007/s00018-006-6295-0 [9] JAIN N K, BARKOWSKI-CLARK S, ALTMAN R, et al. A high density CHO-S transient transfection system: Comparison of ExpiCHO and Expi293 [J]. Protein Expression and Purification, 2017, 134: 38−46. doi: 10.1016/j.pep.2017.03.018 [10] KAUFMAN R J, WASLEY L C, SPILIOTES A J, et al. Coamplification and coexpression of human tissue-type plasminogen activator and murine dihydrofolate reductase sequences in Chinese hamster ovary cells [J]. Molecular and Cellular Biology, 1985, 5(7): 1750−1759. doi: 10.1128/MCB.5.7.1750 [11] 王稳, 王天云. CHO细胞表达系统启动子 [J]. 中国生物化学与分子生物学报, 2019, 35(11):1175−1182.WANG W, WANG T Y. Promoters used in the CHO cell expression system [J]. Chinese Journal of Biochemistry and Molecular Biology, 2019, 35(11): 1175−1182.(in Chinese) [12] WANG D Y, DAI W, WU J, et al. Improving transcriptional activity of human Cytomegalovirus major immediate-early promoter by mutating NF-κB binding sites [J]. Protein Expression and Purification, 2018, 142: 16−24. doi: 10.1016/j.pep.2017.09.008 [13] HO S C L, MARIATI, YEO J H M, et al. Impact of using different promoters and matrix attachment regions on recombinant protein expression level and stability in stably transfected CHO cells [J]. Molecular Biotechnology, 2015, 57(2): 138−144. doi: 10.1007/s12033-014-9809-2 [14] DOVERSKOG M, LJUNGGREN J, ÖHMAN L, et al. Physiology of cultured animal cells [J]. Journal of Biotechnology, 1997, 59(1/2): 103−115. [15] RODRIGUEZ J, SPEARMAN M, HUZEL N, et al. Enhanced production of monomeric interferon-β by CHO cells through the control of culture conditions [J]. Biotechnology Progress, 2008, 21(1): 22−30. doi: 10.1021/bp049807b -








 下载:
下载: