Fitness and Glucosinolate Catabolism-related Gene Expression Upon Feeding on Natural Host Plant of Plutella xylostella Raised from Artificial Diet
-
摘要: 硫代葡萄糖苷硫酸酯酶(GSS)及其修饰因子(SUMF1)是小菜蛾代谢十字花科植物化学防御物质硫苷的关键因子,但其在人工饲料品系小菜蛾适应寄主植物方面的功能研究中鲜有报道。本研究检测了硫苷代谢相关基因GSS和SUMF1在2种人工饲料品系小菜蛾(AD和G88)不同发育阶段的表达模式,发现GSS基因在2种饲料品系间的表达模式较为相似,GSS1和GSS2均在3龄和4龄幼虫中大量表达;SUMF1基因的表达模式则差别较大,未呈现明显规律性。AD和G88品系小菜蛾从初孵幼虫起被转移至萝卜子叶上饲养,与取食人工饲料相比幼虫存活率下降、发育历期延长、蛹重降低;幼虫中肠的GSS1和GSS2表达水平显著下降,AD品系的SUMF1a基因在幼虫中肠的表达亦显著下降。本研究通过探究饲料品系小菜蛾转食寄主植物后适合度与GSS和SUMF1基因表达水平的关系,揭示了硫苷代谢相关基因的表达水平很可能受到植物因子调控,并与人工饲料品系小菜蛾对寄主植物的适应性密切相关。
-
关键词:
- 小菜蛾 /
- 硫代葡萄糖苷硫酸酯酶 /
- 硫酸酯酶修饰因子 /
- 人工饲料品系 /
- 转寄主
Abstract: Glucosinolate sulfatase (GSS) and sulfatase modifying factor 1 (SUMF1) are crucial for the catabolism of Plutella xylostella on the defensive glucosinolates in the cruciferous host plants. However, little information is available on their roles in the adaptation of the strain of P. xylostella raised from an artificial diet when changed to be fed on its natural host plant. The expression patterns of GSSs and SUMF1s at different developmental stages of two artificial diet strains of P. xylostella (i.e., AD and G88) were determined. It was found that the expressions of GSSs were similar between the two strains, with abundant GSS1 and GSS2 expressions at the 3rd and 4th-instar stages, but no apparent patterns observed for SUMF1s. After the newly hatched larvae of AD and G88 were transferred onto the cotyledons of radish plants, the larval survival rates became lower, with longer larval developmental time and lower pupal weight, than their counterparts fed on the original artificial diet. The expression levels of GSS1 and GSS2 in the larval midguts grown on the radish cotyledons decreased significantly; but, that of SUMF1a, only in the midguts of AD strain. It suggested that the expressions of glucosinolate catabolism-related genes in P. xylostella were possibly regulated by the factor(s) in the host plant and closely associated with the adaptability of the insects upon a shifted feeding from a formulated diet to a natural host plant. -
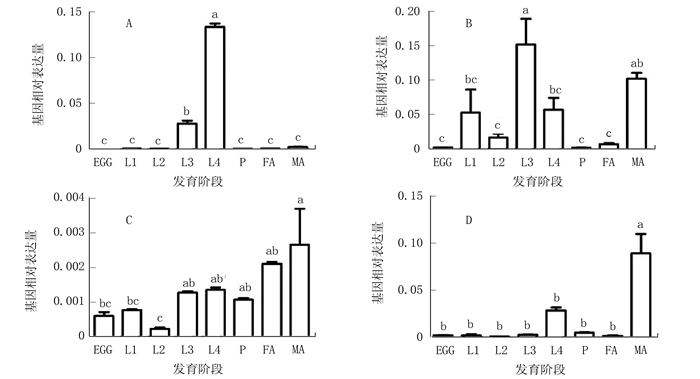
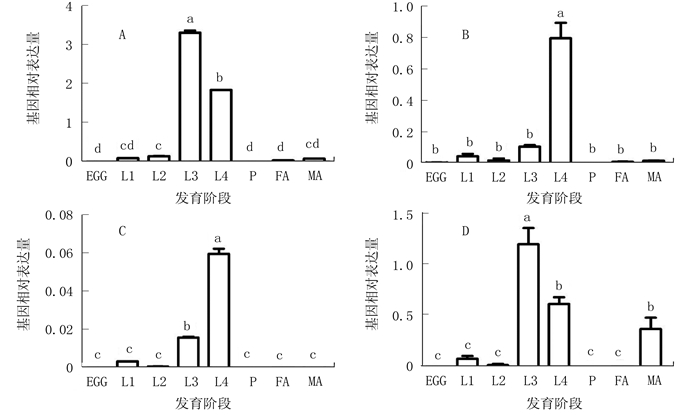
图 1 GSS1和GSS2在不同发育阶段的表达模式
注:A、B、C、D分别为AD-GSS1、G88-GSS1、AD-GSS2、G88-GSS2的表达模式。EGG为卵,L1~L4为幼虫1~4龄期,P为蛹,FA为雌性成虫,MA为雄性成虫。数据以平均值+标准误的形式呈现于图表,不同小写字母表示数据经单因素方差分析后,利用Tukey多重比较检测在0.05水平下存在显著差异。图 2同。
Figure 1. Stage-specific expression patterns of GSS1 and GSS2
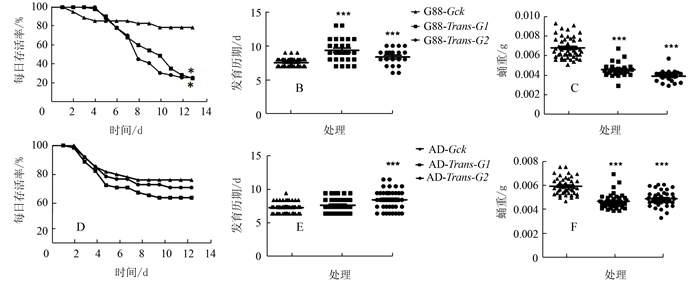
图 3 转换寄主对小菜蛾存活率、发育历期和蛹重的影响
注:A、D分别为G88品系和AD品系幼虫存活率,B、E为幼虫发育历期,C、F为蛹重。G88(AD)-Gck为长期取食人工饲料的小菜蛾G88(AD)品系,G88(AD)-Trans-G1、G2为G88(AD)品系小菜蛾初孵幼虫取食萝卜子叶第一代、第二代。A和D采用Log-rank (Mantel-Cox)分析方法进行两两比较,B、E、C、F采用独立样本t检验;*表示P < 0.05,***表示P < 0.001。
Figure 3. Effect of host shift on survival rate, developmental time and pupal weight of artificial diet stain of P. xylostella
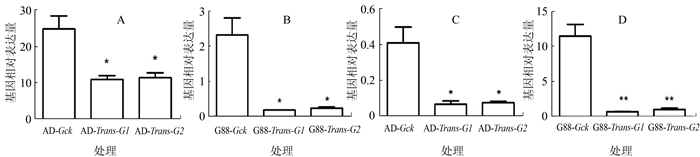
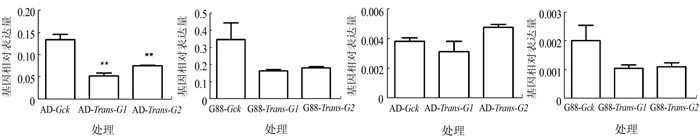
图 4 饲料品系取食萝卜子叶后GSS1和GSS2基因表达模式
注:A、B为GSS1基因,C、D为GSS2基因。AD(G88)-Gck为长期取食人工饲料的AD(G88)小菜蛾品系4龄幼虫中肠,AD (G88)-Trans-G1、G2为AD(G88)品系小菜蛾初孵幼虫取食萝卜子叶第一代、第二代的4龄幼虫中肠,数据分析方法与图 1相同。*P < 0.05,** P < 0.01。图 5同。
Figure 4. Expression patterns of GSS1 and GSS2 in artificial diet stains after feeding on radish cotyledons
表 1 荧光定量PCR引物序列
Table 1. Sequences of primer pairs used for q-PCR
基因 引物对 产物长度/bp 序列 退火温度/℃ GSS1 F: AGGACCCTTGTGAGCTTCGT
R: ACTTGGGGTCAGCGACGT58 129 GSS2 F: CGGACCCTTGCGAGCTGCGA
R: CCCTGGGGTCAGCGGTGA58 129 SUMF1a F: CATAGAAGCGGACAACGAGG
R: TCCACGAACTCACTGAAATC58 102 SUMF1b F: ACATTTCCCAGCCATAACTC
R: ACTCCCAGACATTCCCGACA58 112 RPL32 F:CAATCAGGCCAATTTACCGC
R:CTGGGTTTACGCCAGTTACG58 109 -
[1] AGRAWAL A A. Induced responses to herbivory and increased plant performance[J]. Science, 1998, 279(5354):1201-1202. doi: 10.1126/science.279.5354.1201 [2] HEIL M. Indirect defence via tritrophic interactions[J]. New Phytologist, 2008, 178(1):41-61. doi: 10.1111/j.1469-8137.2007.02330.x [3] EYLES A, BONELLO P, GANLEY R, et al. Induced resistance to pests and pathogens in trees[J]. New Phytologist, 2010, 185(4):893-908. doi: 10.1111/j.1469-8137.2009.03127.x [4] MULLER C. Interactions between glucosinolate-and myrosinase-containing plants and the sawfly Athalia rosae[J]. Phytochemistry Reviews, 2009, 8(1):121-134. doi: 10.1007/s11101-008-9115-3 [5] WINDE I, WITTSTOCK U. Insect herbivore counterada-ptations to the plant glucosinolate-myrosinase system[J]. Phytochemistry, 2011, 72(13):1566-1575. doi: 10.1016/j.phytochem.2011.01.016 [6] KLIEBENSTEIN D J, KROYMANN J, MITCHELL O T. The glucosinolate-myrosinase system in an ecological and evolutionary context[J]. Current Opinion in Plant Biology, 2005, 8(3):264-271. doi: 10.1016/j.pbi.2005.03.002 [7] RAUT J S, BANSODE B S, JADHAV A K, et al. Activity of allyl isothiocyanate and its synergy with fluconazole against Candida albicans biofilms[J]. Journal of Microbiology and Biotechnology, 2017, 27(4):685-693. doi: 10.4014/jmb.1607.07072 [8] BORGEN B, AHUJA I, THANGSTAD O P, et al. 'Myrosin cells' are not a prerequisite for aphid feeding on oilseed rape (Brassica napus) but affect host plant preferences[J]. Plant Biology, 2012, 14(6):894-904. doi: 10.1111/plb.2012.14.issue-6 [9] WITTSTOCK U, AGERBIRK N, STAUBER E J, et al. Successful herbivore attack due to metabolic diversion of a plant chemical defense[J]. Proceedings of the National Academy of Sciences of the U S A, 2004, 101(14):4859-4864. doi: 10.1073/pnas.0308007101 [10] KAZANA E, POPE T W, TIBBLES L, et al. The cabbage aphid:a walking mustard oil bomb[J]. Proceedings of the Royal Society B-Biological Sciences, 2007, 274(1623):2271-2277. doi: 10.1098/rspb.2007.0237 [11] BERAN F, PAUCHET Y, KUNERT G, et al. Phyllotreta striolata flea beetles use host plant defense compounds to create their own glucosinolate-myrosinase system[J]. Proceedings of the National Academy of Sciences of the U S A, 2014, 111(20):7349-7354. doi: 10.1073/pnas.1321781111 [12] RATZKA A, VOGEL H, KLIEBENSTEIN D J, et al. Disarming the mustard oil bomb[J]. Proceedings of the National Academy of Sciences of the U S A, 2002, 99(17):11223-11228. doi: 10.1073/pnas.172112899 [13] YOU M S, YUE Z, HE W Y, et al. A heterozygous moth genome provides insights into herbivory and detoxification[J]. Nature Genetics, 2013, 45(2):220-225. doi: 10.1038/ng.2524 [14] COSMA M P, PEPE S, ANNUNZIATA I, et al. The multiple sulfatase deficiency gene encodes an essential and limiting factor for the activity of sulfatases[J]. Cell, 2003, 113(4):445-456. doi: 10.1016/S0092-8674(03)00348-9 [15] SARDIELLO M, ANNUNZIATA I, ROMA G, et al. Sulfatases and sulfatase modifying factors:an exclusive and promiscuous relationship[J]. Human Molecular Genetics, 2005, 14(21):3203-3217. doi: 10.1093/hmg/ddi351 [16] ZITO E, FRALDI A, PEPE S, et al. Sulphatase activities are regulated by the interaction of sulphatase-modifying factor 1 with SUMF2[J]. EMBO Reports, 2005, 6(7):655-660. doi: 10.1038/sj.embor.7400454 [17] MA X L, HE W Y, CHEN W, et al. Structure and expression of sulfatase and sulfatase modifying factor genes in the diamondback moth, Plutella xylostella[J]. Insect Science, 2017, DOI: 10.1111/1744-7917.12487. [18] SARFRAZ R M, DOSDALL L M, KEDDIE A B, et al. Larval survival, host plant preferences and developmental responses of the diamondback moth Plutella xylostella (Lepidoptera:Plutellidae) on wild brassicaceous species[J]. Entomological Science, 2011, 14(1):20-30. doi: 10.1111/ens.2011.14.issue-1 [19] HOPKINS R J, van DAM N M, van LOON J J A. Role of glucosinolates in insect-plant relationships and multitrophic interactions[J]. Annual Review of Entomology, 2009, 54(1):57-83. doi: 10.1146/annurev.ento.54.110807.090623 [20] AGERBIRK N, OLSEN C E. Glucosinolate structures in evolution[J]. Phytochemistry, 2012, 77(1):16-45. http://cn.bing.com/academic/profile?id=c5a92134385fe420468395331a5cce8f&encoded=0&v=paper_preview&mkt=zh-cn [21] BADENES-PEREZ F R, NAULT B A, SHELTON A M. Manipulating the attractiveness and suitability of hosts for diamondback moth (Lepidoptera:Plutellidae)[J]. Journal of Economic Entomology, 2005, 98(3):836-844. doi: 10.1603/0022-0493-98.3.836 [22] TALEKAR N S, SHELTON A M. Biology, ecology, and management of the diamondback moth[J]. Annual Review of Entomology, 1993, 38(1):275-301. doi: 10.1146/annurev.en.38.010193.001423 [23] FURLONG M J, WRIGHT D J, DOSDALL L M. Diamondback moth ecology and management:problems, progress and prospects[J]. Annual Review of Entomology, 2013, 58(1):517-541. doi: 10.1146/annurev-ento-120811-153605 [24] YANG G, WANG D F, DONG Z Q, et al. Characterization of a myrosinase cDNA from Brassica parachinensis and its defense role against Plutella xylostella after suppression[J]. Insect Science, 2012, 19(4):461-471. doi: 10.1111/ins.2012.19.issue-4 [25] HUANG Y P, CHEN Y, ZENG B, et al. CRISPR/Cas9 mediated knockout of the abdominal-A homeotic gene in the global pest, diamondback moth (Plutella xylostella)[J]. Insect Biochemistry and Molecular Biology, 2016, 75:98-106. doi: 10.1016/j.ibmb.2016.06.004 [26] HUANG Y P, WANG Y, ZENG B, et al. Functional characterization of Pol Ⅲ U6 promoters for gene knockdown and knockout in Plutella xylostella[J]. Insect Biochemistry and Molecular Biology, 2017, 89:71-78. doi: 10.1016/j.ibmb.2017.08.009 -






 下载:
下载: