Effects of Bt Rice on the Structure of Culturable Bacteria in the Gut of Nilaparvata lugens Nymphae
-
摘要:
目的 探究Bt水稻对褐飞虱若虫肠道可培养细菌组成结构的影响,为转基因水稻的安全性评价提供科学依据。 方法 利用传统微生物纯培养法分别从取食非转基因(CK)水稻和转crylAb抗虫(Bt)水稻的褐飞虱肠道内分离细菌,利用16s rDNA对分离菌株进行鉴定,比较分析转基因水稻对褐飞虱若虫肠道微生物群落结构的影响。 结果 研究结果显示在取食CK水稻和Bt水稻的褐飞虱肠道内各分离获得7株细菌。取食CK水稻的褐飞虱肠道细菌分别属于3个门5个属;而取食Bt水稻的褐飞虱肠道细菌分别属于2个门4个属;分离获得频率最高的是变形菌门细菌Proteobateria,其中取食两种水稻均能分离获得变形菌门的泛菌属Pantoea,以及厚壁菌门Firmicutes的葡萄球菌属Staphylococcus等2个属的细菌。取食CK水稻的褐飞虱肠道内分离获得变形菌门的草螺菌属Herbaspirillum和不动杆菌属Acinetobacter,以及放线菌门的微杆菌属Microbacterium等3个特异性细菌属;取食Bt水稻的褐飞虱肠道内分离得到变形菌门的肠杆菌属Enterobacter和伯克氏菌属Burkholderia等2个特异性细菌属。 结论 取食非转基因水稻和转基因水稻的褐飞虱肠道可培养细菌组成存在一定的差异。本研究为后续深入研究褐飞虱肠道共生细菌的功能,以及转基因水稻对非靶标生物肠道细菌的影响提供了材料和基础。 Abstract:Objective The present study aims to explore the effects of Bt rice on gut bacterial structure of the Nilaparvata lugens nymphae and to provide evidence for rational safety assessment of Bt rice. Method Gut bacteria from BPH feeding on non-transgenic rice (CK) and transgenic rice (Bt) were isolated using traditional culture methods, and were identified using 16s rDNA. The composition of isolates was compared between these two treatments. Results The results showed that seven bacterial strains were isolated from the gut of N. lugens feeding on either CK or Bt rice. Three phyla and five genera of the gut bacteria were identified from N. lugens feeding on CK rice, while two phyla and four genera of the gut bacteria from N. lugens feeding on Bt rice. The taxa of bacteria identified from the guts of N. lugens either feeding on CK rice or Bt rice were Proteobateria (including the genus of Pantoea) and Firmicutes (including the genus of Staphylococcus). Herbaspirillum Acinetobacter and Microbacterium were specific genera from the CK treatment, while Enterobacter, and Burkholderia from the Bt treatment. Conclusion The composition of culturable bacteria in the N. lugens gut was different beween the CK treatment and the Bt treatment. The findings provide an important foundation for further studies on the function of symbiotic bacteria in N. lugens, and the effects of transgenic rice on the gut bacteria of non-target species. -
Key words:
- Nilaparvata lugens /
- genetically modified rice /
- gut bacteria /
- non-target effect /
- biosafety
-
图 3 取食非转基因水稻的褐飞虱肠道细菌16S rDNA PCR产物电泳图
注:M泳道为100~5 000 bp DNA marker,1~14泳道分别为7株菌株CK1~CK7的2次重复,15泳道为阴性对照。
Figure 3. Gel electrophoresis of 16S rDNA PCR products of gut bacteria from Nilaparvata lugens feeding on the non-transgenic rice
Note: Lane M is DNA marker(100-5 000 bp), Lane 1-14 are PCR products of CK1 to CK7 with 1 repeat of each strain, respectively, Lane 15 is negative control.
图 4 取食转基因水稻褐飞虱肠道细菌16S rDNA PCR产物电泳图
注:M泳道为100~5 000 bp DNA marker,1~14泳道分别为7株菌株Bt1~Bt7的2次重复,15泳道为阴性对照。
Figure 4. Gel electrophoresis of 16S rDNA PCR products of gut bacteria from N. lugens feeding on the transgenic rice
Note: Lane M is DNA marker (100-5 000 bp), Lane 1-14 are PCR products of Bt1 to Bt7 with 1 repeat of each strain, respectively, Lane 15 is negative control.
图 5 取食非转基因水稻的褐飞虱肠道细菌16S rDNA系统进化分析
注:CK1~7序列分离自取食非转基因水稻褐飞虱肠道,分支间的数值是基于bootstrap验证的自展检验值(100次重复),刻度标尺表示遗传距离为0.5。
Figure 5. Phylogenetic analysis based on 16S rDNA sequences from the gut bacteria of N. lugens feeding on the non-transgenic rice
Note: The sequences of CK1-7 were isolated from N. lugens gut bacteria feeding on the non-transgenic rice. The bootstrap test (100 replicates) was shown next to the branches. The scale bar represents genetic distance of 0.5.
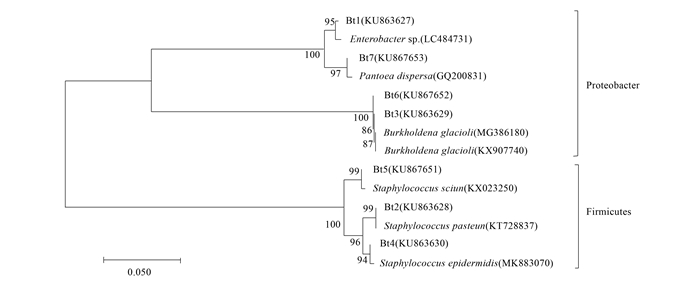
图 6 取食转基因水稻的褐飞虱肠道细菌16S rDNA系统进化分析
注:Bt1~7序列分离自取食转基因水稻褐飞虱肠道,分支间的数值是基于bootstrap验证的自展检验值(100次重复),刻度标尺表示遗传距离为0.05。
Figure 6. Phylogenetic analysis based on 16S rDNA sequences from the gut bacteria of N. lugens feeding on the transgenic rice
Note: The sequences of Bt1-7 were isolated from N. lugens gut bacteria feeding on the transgenic rice. The bootstrap test (100 replicates) was shown next to the branches. The scale bar represents genetic distance of 0.05.
表 1 褐飞虱肠道细菌的16S rDNA序列的Blast比对结果
Table 1. Blast-based sequence alignments of 16S rDNA from the gut bacteria from N. lugens
菌株
Strain ID登录号
GenBank Accession No.相似性最高菌株(GenBank登录号)
Most closely related hit in GenBank (GenBank Accession No.)相似性
Similarity/%分类地位(门)
Taxonomy (Phylum)CK1 KU863620 Pantoea ananatis (KT380684) 99 Proteobacteria CK2 KU863621 Staphylococcus sciuri (JX134627) 99 Firmicutes CK3 KU863622 Acinetobacter baylyi (KJ372731) 100 Proteobacteria CK4 KU863623 Microbacterium laevaniformans (JQ229794) 99 Actinobacteria CK5 KU863624 Pantoea sp. (LC484790) 99 Proteobacteria CK6 KU863625 Staphylococcus saprophyticus (MK208692) 100 Firmicutes CK7 KU863626 Herbaspirillum sp. (KM253161) 99 Proteobacteria Bt1 KU863627 Enterobacter sp. (LC484731) 99 Proteobacteria Bt2 KU863628 Staphylococcus pasteuri (KT728837) 100 Firmicutes Bt3 KU863629 Burkholderia gladioli (MG386180) 99 Proteobacteria Bt4 KU863630 Staphylococcus epidermidis (MK883070) 99 Firmicutes Bt5 KU867651 Staphylococcus sciuri (KX023250) 99 Firmicutes Bt6 KU867652 Burkholderia gladioli (KX907740) 99 Proteobacteria Bt7 KU867653 Pantoea dispersa (GQ200831) 99 Proteobacteria -
[1] LIU Q, HALLERMAN E, PENG Y, et al. Development of Bt rice and Bt maize in China and their efficacy in target pest control[J]. International Journal of Molecular Sciences, 2016, 17(10):1561. doi: 10.3390/ijms17101561 [2] LI Y, HALLERMAN E M, LIU Q, et al. The development and status of Bt rice in China[J]. Plant Biotechnology Journal, 2016, 14(3):839-848. doi: 10.1111/pbi.12464 [3] ZHANG F, ZHU L, HE G C. Differential gene expression in response to brown planthopper feeding in rice[J]. Journal of Plant Physiology, 2004, 161(1):53-62. doi: 10.1078/0176-1617-01179 [4] WEI T, UEHARA-ICHIKI T, MIYAZAKI N, et al. Association of rice gall dwarf virus with microtubules is necessary for viral release from cultured insect vector cells[J]. Journal of Virology, 2009, 83(20):10830-10835. doi: 10.1128/JVI.01067-09 [5] JIAO Y, HU X, PENG Y, et al. Bt rice plants may protect neighbouring non-Bt rice plants against the striped stem borer, Chilo suppressalis[J]. Proceedings of the Royal Society B-Biological Sciences, 2018, 285(1883):20181283. doi: 10.1098/rspb.2018.1283 [6] WANG X, LIU Q, MEISSLE M, et al. Bt rice could provide ecological resistance against nontarget planthoppers[J]. Plant Biotechnology Journal, 2018, 16(10):1748-1755. doi: 10.1111/pbi.12911 [7] HAN L, JIANG X, PENG Y. Potential resistance management for the sustainable use of insect-resistant genetically modified corn and rice in China[J]. Current Opinion in Insect Science, 2016, 15:139-143. doi: 10.1016/j.cois.2016.04.004 [8] 刘勇波, 李俊生, 赵彩云, 等.转基因水稻基因流的发生与生态学后果[J].应用昆虫学报, 2012, 23(6):1713-1720. http://d.old.wanfangdata.com.cn/Periodical/yystxb201206039LIU Y B, LI J S, ZHAO C Y, et al. Occurrence and ecological consequences of transgenic rice gene flow:A review[J]. Chinese Journal of Applied Entomology, 2012, 23(6):1713-1720. (in Chinese) http://d.old.wanfangdata.com.cn/Periodical/yystxb201206039 [9] 刘雨芳.转Bt抗虫稻对地上非靶标节肢动物的生态风险性[J].应用昆虫学报, 2014, 51(5):1133-1142. http://d.old.wanfangdata.com.cn/Periodical/kczs201405001LIU Y F. An overview of the potential ecological risk of insect-resistant transgenic Bt rice on non-target arthropods aboveground in fields[J]. Chinese Journal of Applied Entomology, 2014, 51(5):1133-1142. (in Chinese) http://d.old.wanfangdata.com.cn/Periodical/kczs201405001 [10] 高秀云, 田俊策, 陈洋, 等. Biolog-Eco方法检测转cry1Ab粳稻对褐飞虱肠道微生物多样性的影响[J].植物保护学报, 2008, 35(4):327-331. doi: 10.3321/j.issn:0577-7518.2008.04.008GAO X Y, TIAN J C, CHEN Y, et al. Impact evaluation of transgenic cry1Ab japonica rice on the diversity of intesinal microbial community in the brown planthopper, Nilaparvata lugens using Biolog-Eco method[J]. Acta Phytophylacica Sinica, 2008, 35(4):327-331. (in Chinese) doi: 10.3321/j.issn:0577-7518.2008.04.008 [11] AKBAR N, SIDDIQUI R, SAGATHEVAN K A, et al. Gut bacteria of animals/pests living in polluted environments are a potential source of antibacterials[J]. Applied Microbiology and Biotechnology, 2019, 103(10):3955-3964. doi: 10.1007/s00253-019-09783-2 [12] ZHOU J, DUAN J, GAO M, et al. Diversity, roles, and biotechnological applications of symbiotic microorganisms in the gut of termite[J]. Current Microbiology, 2019, 76(6):755-761. doi: 10.1007/s00284-018-1502-4 [13] BRODERICK N A, ROBINSON C J, McMAHON M D, et al. Contributions of gut bacteria to Bacillus thuringiensis-induced mortality vary across a range of Lepidoptera[J]. BMC Biology, 2009, 7:11. doi: 10.1186/1741-7007-7-11 [14] DILLON R J, VENNARD C T, BUCKLING A, et al. Diversity of locust gut bacteria protects against pathogen invasion[J]. Ecology Letters, 2005, 8(12):1291-1298. doi: 10.1111/j.1461-0248.2005.00828.x [15] GHANIM M, KONTSEDALOV S. Susceptibility to insecticides in the Q biotype of Bemisia tabaci is correlated with bacterial symbiont densities[J]. Pest Management Science, 2009, 65(9):939-942. doi: 10.1002/ps.1795 [16] DILLON R J, VENNARD C T, CHARNLEY A K. A Note:Gut bacteria produce components of a locust cohesion pheromone[J]. Journal of Applied Microbiology, 2002, 92(4):759-763. doi: 10.1046/j.1365-2672.2002.01581.x [17] LANE D J. 16S/23S rRNA sequencing. In:Stackebrandt E, Goodfellow M. (Eds.), Nucleic acid techniques in bacterial systematics[M]. Wiley, Chichester, 1991:371-375. [18] HUANG X, HE J, SUN J, et al. Isolation and characterization of a metsulfuron-methyl degrading bacterium Methylopila sp. S113[J]. International Biodeterioration & Biodegradation, 2007, 60(3):152-158. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=0d99e689ac4820f5bedba9f0884fca54 [19] TAMURA K, STECHER G, PETERSON D, et al. MEGA6:Molecular evolutionary genetics analysis version 6.0[J]. Molecular Biology and Evolution, 2013, 30(12):2725-2729. doi: 10.1093/molbev/mst197 [20] SAITOU N, NEI M. The neighbor-joining method:a new method for reconstructing phylogenetic trees[J]. Molecular Biology and Evolution, 1987, 4(4):406-425. http://cn.bing.com/academic/profile?id=dd63fd2642d38f24d220713d22692d24&encoded=0&v=paper_preview&mkt=zh-cn [21] 陆剑飞, 黄国洋.浙江省褐飞虱暴发成灾的原因与治理对策[J].农药科学与管理, 2006, 27(1):42-43. doi: 10.3969/j.issn.1002-5480.2006.01.014LU J F, HUANG G Y. Causes and control strategies of the outbreak of brown planthopper in Zhejiang Province[J]. Pesticide Science and Administration, 2006, 27(1):42-43. (in Chinese) doi: 10.3969/j.issn.1002-5480.2006.01.014 [22] 相辉, 黄勇平.肠道微生物与昆虫的共生关系[J].昆虫知识, 2008, 45(5):687-693. doi: 10.3969/j.issn.0452-8255.2008.05.003XIANG H, HUANG Y P. Symbiosis between gut microbiota and insects[J]. Chinese Bulletin of Entomology, 2008, 45(5):687-693. (in Chinese) doi: 10.3969/j.issn.0452-8255.2008.05.003 [23] DILLON R J, DILLON V M. The gut bacteria of insects:Nonpathogenic interactions[J]. Annual Review of Entomology, 2004, 49:71-92. doi: 10.1146/annurev.ento.49.061802.123416 [24] RAJAGOPAL R. Beneficial interactions between insects and gut bacteria[J]. Indian Journal of Microbiology, 2009, 49(2):114-119. doi: 10.1007/s12088-009-0023-z [25] KIKUCHI Y, HOSOKAWA T, FUKATSU T. Insect-microbe mutualism without vertical transmission:a stinkbug acquires a beneficial gut symbiont from the environment every generation[J]. Applied and Environmental Microbiology, 2007, 73(13):4308-4316. doi: 10.1128/AEM.00067-07 [26] HUI X, WEI G F, JIA S, et al. Microbial communities in the larval midgut of laboratory and field populations of cotton bollworm (Helicoverpa armigera)[J]. Canadian Journal of Microbiology, 2006, 52(11):1085-1092. doi: 10.1139/w06-064 [27] 相辉, 李木旺, 赵勇, 等.家蚕幼虫中肠细菌群落多样性的PCR-DGGE和16S rDNA文库序列分析[J].昆虫学报, 2007, 50(3):222-233. doi: 10.3321/j.issn:0454-6296.2007.03.003XIANG H, LI B W, ZHAO Y, Bacterial community in midguts of the silkworm larvae estimated by PCR DGGE and 16S rDNA gene library analysis[J]. Acta Entomologica Sinica, 2007, 50(3):222-233. (in Chinese) doi: 10.3321/j.issn:0454-6296.2007.03.003 [28] 夏晓峰, 郑丹丹, 林海兰, 等.小菜蛾幼虫中肠细菌的分离鉴定[J].应用昆虫学报, 2013, 50(3):770-776. http://d.old.wanfangdata.com.cn/Periodical/kczs201303027XIA X F, ZHENG D D, LIN H L, et al. Isolation and identification of bacteria from the larval midgut of the diamondback moth, Plutella xylostella[J]. Chinese Journal of Applied Entomology, 2013, 50(3):770-776. (in Chinese) http://d.old.wanfangdata.com.cn/Periodical/kczs201303027 [29] 范忠原, 李光玉, 刘晗璐.宏基因组学在动物胃肠道微生物研究中的应用[J].中国畜牧兽医, 2014, 41(12):116-122. http://d.old.wanfangdata.com.cn/Periodical/zgxmsy201412022FAN Z Y, LI G Y, LIU H L. Research progress on application of metagenomics in the animal gastrointestinal tract microbiota[J]. China Animal Husbandry and Veterinary Medicime, 2014, 41(12):116-122. (in Chinese) http://d.old.wanfangdata.com.cn/Periodical/zgxmsy201412022 [30] 王天召, 王正亮, 朱杭锋, 等.基于高通量测序的褐飞虱肠道微生物多样性分析[J].昆虫学报, 2019, 62(3):323-333. http://d.old.wanfangdata.com.cn/Periodical/kcxb201903006WANG T Z, WANG Z L, ZHU H F, et al. Analysis of the gut microbial diversity of the brown planthopper, Nilaparvata lugens (Hemiptera:Delphacidae) by high-throughput sequencing[J]. Acta Entomologica Sinica, 2019, 62(3):323-333. (in Chinese) http://d.old.wanfangdata.com.cn/Periodical/kcxb201903006 -







 下载:
下载: