Mapping of Early Senescence-related OsBRCA1 in Rice by BSA-seq Technique
-
摘要:
目的 水稻早衰突变体是研究水稻衰老机制的良好载体。定位和克隆水稻早衰相关基因,有助于理解水稻早衰的遗传规律和分子机制,为相关基因的作用机制研究奠定基础。 方法 用EMS化学诱变处理粳稻云引,获得一个稳定遗传的早衰突变体w14,与日本晴杂交构建F2分离群体,并在群体中各选择100个隐性单株和显性单株的DNA等量混合,利用BSA-seq分析两个DNA池之间的差异位点,定位水稻早衰相关基因。 结果 突变体w14的早衰表型受单隐性基因控制,对两个DNA池测序结果进行单基因定位和突变基因的鉴定,发现单基因主峰位于Chr3,候选区间为 Chr3:27.5~29.5 Mb,进一步在目标区域内找到2个符合条件的候选因果变异。 结论 候选基因 LOC_Os03g49210突变位点是由野生型的C突变为T,且该突变位于候选基因的第2 外显子上,属于错义突变,造成了该基因编码的第20 个氨基酸由T(苏氨酸)变成I(异亮氨酸),从而可能导致了基因功能的改变,因此,将该基因定为本研究的候选基因,因与人类BRCA1同源,命名为 OsBRCA1 。 Abstract:Objective Molecular mechanism of rice senescence was studied by locating and cloning the specific gene from a mutant artificially induced with early senescence. Methods Japonica rice Yunyin was treated with EMS to artificially induce a genetically stable early senescence w14. The mutant was crossed with Nipponbare to construct an isolated F2 population. Subsequently, equal parts of the DNA of 100 recessive and dominant plants in the population were mixed. The differential loci between these two DNA pools were analyzed by BSA-seq to locate the target early senescence-related gene. Results The early senescence phenotype of w14 was found to be controlled by a single recessive gene. The mutagenic gene mapped and identified by the specific sequence showed the main peak located in Chr 3 and the candidate interval Chr 3: 27.5–29.5 Mb. In the region, two qualified candidate causal variants were found. Conclusion The mutation site of the candidate gene Loc_ Os03g49210 located in the 2nd exon was determined to have changed in the wild-type from C to T. It was a non-synonymous mutation that resulted in the 20th amino acid encoded by the gene changed from T (threonine) to I (isoleucine) and led to the functional alternation. Being homologous with human BRCA1, the target gene was named OsBRCA1. -
Key words:
- rice /
- mutant /
- early senescence /
- gene clone /
- BSA-seq
-
表 1 用于 OsBRCA1基因组全长分段扩增的引物序列
Table 1. Primer sequences for genome full-length fragment amplification on OsBRCA1
序号
Number标记
Marker上游引物
Forward primer(5′-3′)下游引物
Reverse primer(5′-3′)1 49210-1 AATACCATATCGCCGTTTTCTT AACTTTTCCATCACATCGTTCTAA 2 49210-2 TTGCCCGCATAATGTGACTG AAGACTGGAGACTTGGGAGGTG 3 49210-3 TTTGCTATGCTGAACTCCCG TCCCTTCCACCTTCTTCTTTG 4 49210-4 GATATGTTGGGTCATTTTGGAGC TCAAGTTTGTAAGTTGGTGGTCG 5 49210-5 CAAGGTCCACAGGAAGAAGGT AAGATTGTTGCACTCAAAGAAATG 6 49210-6 CTGGCTAACTCCACATGCTCTT TTCCACTGGCCTACCTCACG 7 49210-7 CCAACTAATGAACCTGGAGCG CAATATGTGCAACCAAAATGTGAA 8 49210-8 GGGCAGCCTTCACTAATGACA TTACGCTGAAACAGGGAAATG 9 49210-9 GCTGAGGATGGAATACGAAGGA GGTGGAGGGAAATCGAGGAG 表 2 测序数据和测序质量的统计分析
Table 2. Statistical analysis on sequencing data and quality
样本
Sample原始数据
Raw base/bp过滤数据
Clean base/bp比例
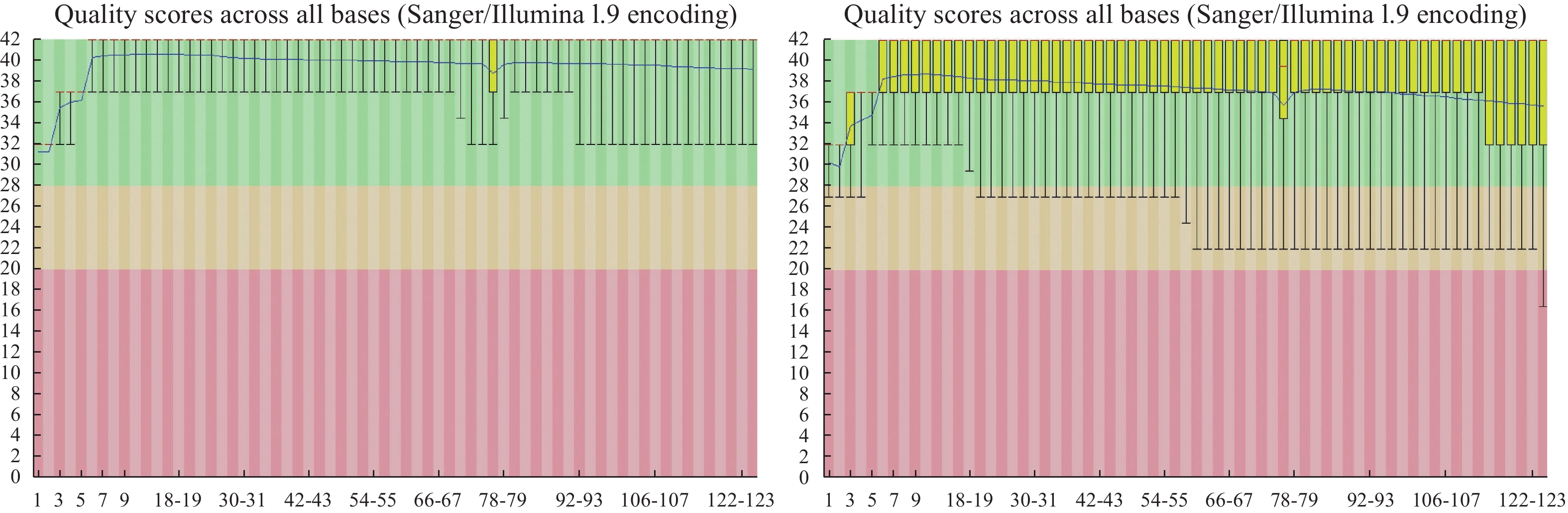
Rate/%Q20/% Q30/% GC/% w14 7 057 752 750 6 731 469 250 95.38 96.10 91.15 44.27 隐性基因池 Recessive gene pool 12 841 555 750 12 418 982 750 96.71 96.00 91.04 44.43 显性基因池 Dominant gene pool 9 239 019 250 8 888 096 500 96.20 95.96 91.00 42.56 注: Q20及Q30:Phred数值大于20、30的碱基占总体碱基的百分比,其中,Phred=-10log10(e),e为错误率。
Note: Q20 and Q30: percentages of bases with Phred values greater than 20 and 30, respectively, in total number of bases; Phred=-10log10(e); e: error rate.表 3 目标染色体的因果变异鉴定结果
Table 3. Causal variant identification on target chromosome
染色体
Chromosome物理位置
Physical location野生型
Wild type隐性池
Hidden pool显性池
Dominant pool野生型参考基因组
WT-IRGSP5Chr3 27726166 1/1∶8∶1∶7 1/1∶41∶0∶41 0/1∶25∶19∶6 0/0∶1∶1∶0 Chr3 28129631 0/1∶11∶2∶9 0/1∶39∶2∶37 0/1∶31∶20∶11 0/0∶13∶13∶0 注:表中数据0/0表示参考基因组序列的纯合,1/1表示变异序列的纯合。
Note: * 0/0 and 1/1 represent homozygous of reference and variant genome sequence, respectively.表 4 利用snpEff对候选因果变异进行注释的结果
Table 4. Annotated candidate causal variant by snpEff
差异位点
Variable site差异位点1
Variable site 1差异位点2
Variable site 2物理位置 Physical location 27726166 28129631 突变基因 Mutant gene LOC_Os03g48626 LOC_Os03g49210 变异结果 Consequence 变异发生在内含子中 错义突变aCc(T) → aTc(I)由于C变异为T,导致第20个氨基酸T变为I 基因功能注释 Functional annotation 表达蛋白 含有BRCA1 C末端结构域的蛋白质 -
[1] LIM P O, KIM H J, NAM H G. Leaf senescence [J]. Annual Review of Plant Biology, 2007, 58: 115−136. doi: 10.1146/annurev.arplant.57.032905.105316 [2] BECK C I, ULRICH T H. Environmental release permits [J]. Bio/Technology, 1993, 11(12): 1524−1528. [3] SWAMINATHAN M S. Towards a hunger-free India [J]. The Indian Journal of Nutrition and Dietetics, 1999, 36(4): 108−117. [4] DAI L Y, LIU X L, XIAO Y H, et al. Recent advances in cloning and characterization of disease resistance genes in rice [J]. Journal of Integrative Plant Biology, 2007, 49(1): 112−119. doi: 10.1111/j.1744-7909.2006.00413.x [5] PROJECT I R G S. The map-based sequence of the rice genome [J]. Nature, 2005, 436(7052): 793−800. doi: 10.1038/nature03895 [6] YOSHIDA S. Molecular regulation of leaf senescence [J]. Current Opinion in Plant Biology, 2003, 6(1): 79−84. doi: 10.1016/S1369526602000092 [7] 王备芳, 陈玉宇, 张迎信, 等. 水稻早衰突变体es5的鉴定及其突变基因的精细定位 [J]. 中国农业科学, 2018, 51(4):613−625. doi: 10.3864/j.issn.0578-1752.2018.04.002WANG B F, CHEN Y Y, ZHANG Y X, et al. Identification and fine mapping of an early senescent leaf mutant Es5 in Oryza sativa L [J]. Scientia Agricultura Sinica, 2018, 51(4): 613−625.(in Chinese) doi: 10.3864/j.issn.0578-1752.2018.04.002 [8] SAKURABA Y, RAHMAN M L, CHO S H, et al. The rice faded green leaf locus encodes protochlorophyllide oxidoreductase B and is essential for chlorophyll synthesis under high light conditions [J]. The Plant Journal:for Cell and Molecular Biology, 2013, 74(1): 122−133. doi: 10.1111/tpj.12110 [9] LIN A H, WANG Y Q, TANG J Y, et al. Nitric oxide and protein S-nitrosylation are integral to hydrogen peroxide-induced leaf cell death in rice [J]. Plant Physiology, 2011, 158(1): 451−464. [10] LIANG C Z, WANG Y Q, ZHU Y N, et al. OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice [J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(27): 10013−10018. doi: 10.1073/pnas.1321568111 [11] KONG Z S, LI M N, YANG W Q, et al. A novel nuclear-localized CCCH-type zinc finger protein, OsDOS, is involved in delaying leaf senescence in rice [J]. Plant Physiology, 2006, 141(4): 1376−1388. doi: 10.1104/pp.106.082941 [12] 朱永生, 蒋家焕, 蔡秋华, 等. 水稻早衰突变体w14的生理学特性分析及其基因的精细定位 [J]. 科学通报, 2021, 66(32):4144−4156. doi: 10.1360/TB-2021-0012ZHU Y S, JIANG J H, CAI Q H, et al. Analysis of physiological characteristics of early leaf senescence mutant w14 and its gene mapping for rice [J]. Chinese Science Bulletin, 2021, 66(32): 4144−4156.(in Chinese) doi: 10.1360/TB-2021-0012 [13] 梁廷敏, 郭新睿, 陈子强, 等. 水稻材料IR65482抗稻瘟病基因鉴定与定位 [J]. 分子植物育种, 2018, 16(13):4308−4313.LIANG T M, GUO X R, CHEN Z Q, et al. Identification and mapping of a blast disease resistance gene in rice line IR65482 [J]. Molecular Plant Breeding, 2018, 16(13): 4308−4313.(in Chinese) [14] SUN J, YANG L M, WANG J G, et al. Identification of a cold-tolerant locus in rice (Oryza sativa L. ) using bulked segregant analysis with a next-generation sequencing strategy [J]. Rice (New York, N Y ), 2018, 11(1): 24. [15] SALUNKHE A S, POORNIMA R, PRINCE K S J, et al. Fine mapping QTL for drought resistance traits in rice (Oryza sativa L. ) using bulk segregant analysis [J]. Molecular Biotechnology, 2011, 49(1): 90−95. doi: 10.1007/s12033-011-9382-x [16] LAFARGE S, MONTANÉ M H. Characterization of Arabidopsis thaliana ortholog of the human breast cancer susceptibility gene 1: AtBRCA1, strongly induced by gamma rays [J]. Nucleic Acids Research, 2003, 31(4): 1148−1155. doi: 10.1093/nar/gkg202 [17] BLOCK-SCHMIDT A S, DUKOWIC-SCHULZE S, WANIECK K, et al. BRCC36A is epistatic to BRCA1 in DNA crosslink repair and homologous recombination in Arabidopsis thaliana [J]. Nucleic Acids Research, 2010, 39(1): 146−154. [18] TRAPP O, SEELIGER K, PUCHTA H. Homologs of breast cancer genes in plants [J]. Frontiers in Plant Science, 2011, 2: 19. [19] EINSET J, COLLINS A R. DNA repair after X-irradiation: Lessons from plants [J]. Mutagenesis, 2014, 30(1): 45−50. -







 下载:
下载:




