Identification and Copy Number of As6G-FFT in Transgenic Tobacco Plant
-
摘要:
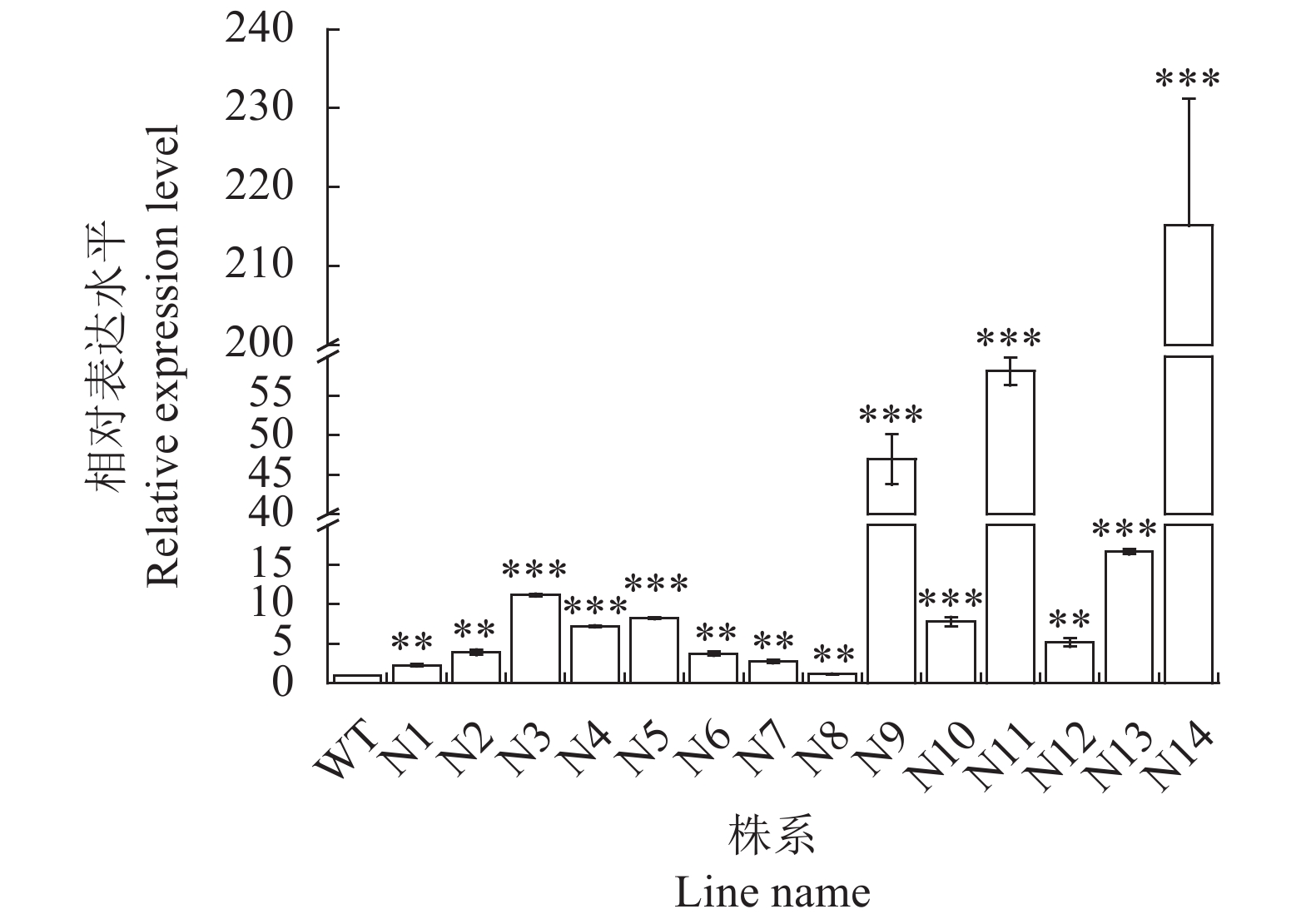
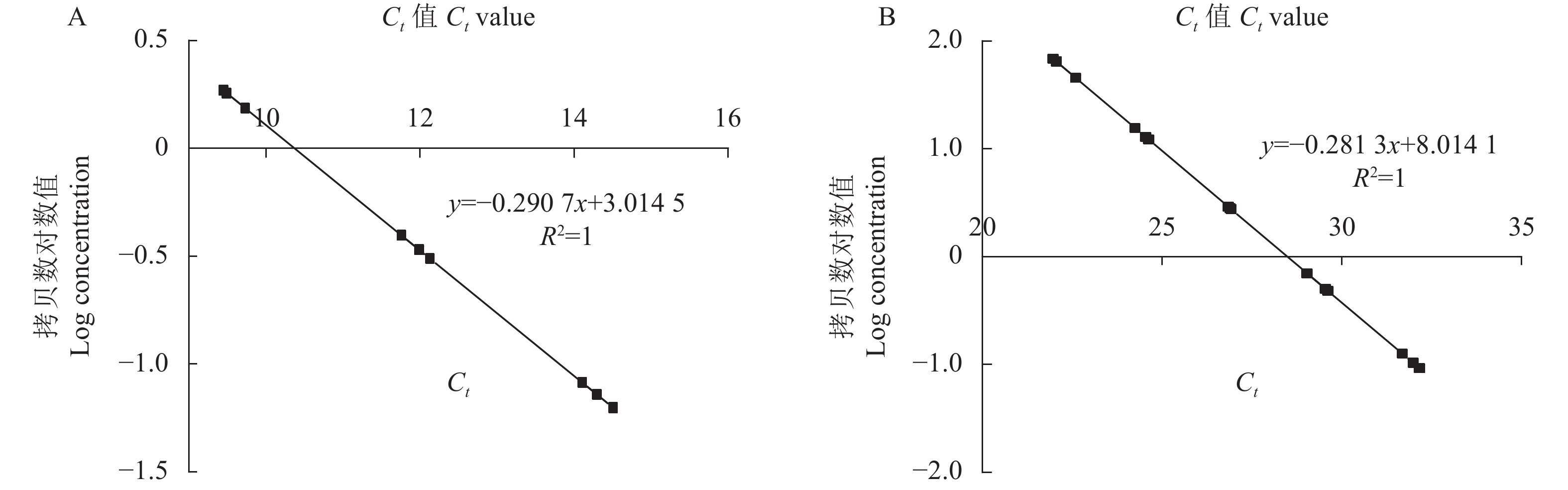
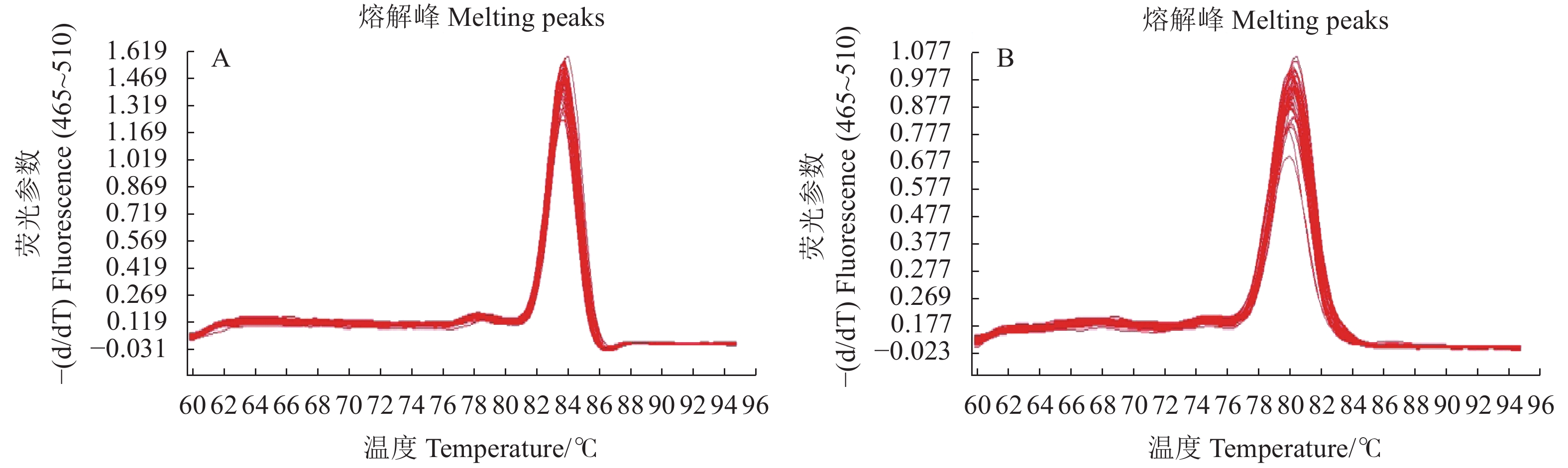
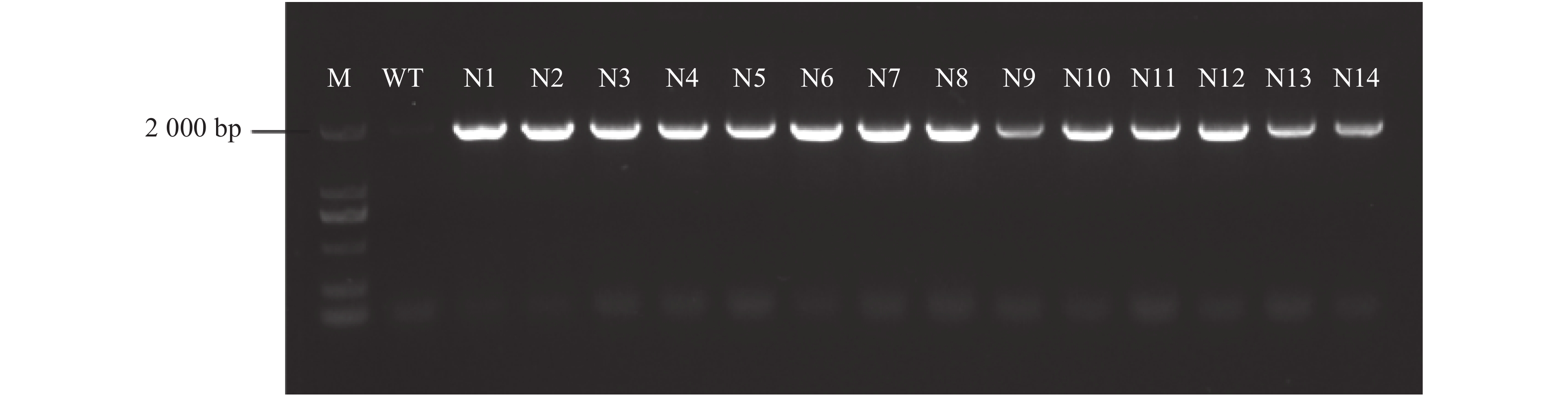
目的 为验证转As6G-FFT烟草的基因功能,筛选稳定遗传的阳性株系材料,以建立基于SYBR Green的实时荧光定量PCR的转基因拷贝数检测方法。 方法 利用PCR检测、实时荧光定量PCR(Real-time quantitative PCR,qRT-PCR)技术及生理指标分析鉴定转As6G-FFT基因阳性烟草植株,并利用基于SYBR Green的实时荧光定量PCR鉴定阳性转基因烟草中As6G-FFT基因的拷贝数。 结果 (1)基于PCR检测,14个转基因烟草叶片均能扩增出目的片段,表明14个株系中均已成功转入目的基因As6G-FFT;(2)14个转基因株系中As6G-FFT基因表达量呈极显著(P<0.01)或极其显著上升(P <0.001),其中6个株系的表达量呈极其显著升高(P <0.001);且其表达量与野生型相比最高提高215.13倍;(3)基于生理指标,测定转As6G-FFT基因烟草的果聚糖含量,发现14个转基因株系中果聚糖含量呈极显著(P <0.01)或极其显著上升(P <0.001),其中13个株系的果聚糖含量极其显著升高(P <0.001);且其果聚糖含量与野生型相比最高提高10.47倍;(4)基于SYBR Green实时荧光定量PCR构建As6G-FFT和NtACT基因的标准曲线,分别为y=−0.290 7x+3.014 5和y=−0.2813x+8.0141,R2均为1;在检测的14个转基因株系中As6G-FFT基因拷贝数为1~3,其中1、2和3拷贝的单株数分别占总数的35.7%、50.0%和14.3%。 结论 本研究从DNA、RNA和生理水平综合进行阳性转基因烟草的鉴定,鉴定结果更为准确。此外,还建立了基于SYBR Green实时荧光定量PCR的转基因烟草中外源As6G-FFT基因拷贝数检测方法,可用于快速、高效地估算转基因烟草中外源基因拷贝数,为后续获得稳定遗传材料提供筛选依据。 -
关键词:
- As6G-FFT基因 /
- 转基因烟草 /
- 阳性鉴定 /
- 拷贝数
Abstract:Objective Functions, identification, and copy number of As6G-FFT in the transgenic tobacco plants were studied. Method PCR, qRT-PCR, and physiological analysis were performed to confirm the transgenic tobacco plants being As6G-FFT-positive and elucidate the functions of the gene. SYBR green-based qRT-PCR was applied to determine the copy number of the gene in the transgenic plant. Result (1) The target fragment was amplified on the leaves of 14 tobacco plants by PCR assuring a successful transfer of As6G-FFT. (2) In varying degrees, the gene expressions in the 14 transgenic lines were higher than in the wild-type. Six of the lines were extremely significantly higher than the wild-type counterpart, with an accumulation topped 215.13-fold. (3) The fructan contents were higher in the leaves of the transgenic than the wild-type plants. Thirteen of the transgenic lines contained extremely significantly more fructan than the wild-type with the highest accumulation of 10.47-fold. (4) With correlation coefficients of 1, the SYBR green-based qRT-PCR standard curves of y=−0.2907x+3.0145 was obtained for As6G-FFT and y=−0.2813x+8.0141 for NtACT. Of the 14 transgenic lines, 35.7% contained only one of the gene, 50.0% had 2, and 14.3% 3 copies. Conclusion Transgenic tobacco plants with As6G-FFT were identified based on the DNA, RNA, and physiological aspects. The SYBR green-based qRT-PCR method rapidly and efficiently determined the number of exogenous As6G-FFT transferred into the plants and could be a convenient tool for screening and acquisition of stable genetic materials. -
Key words:
- As6G-FFT /
- transgenic tobacco /
- positive identification /
- copy number
-
表 1 引物序列
Table 1. Primer sequence
引物
Primer引物序列
(5′-3′) Primer sequence目的
PurposeAs6G-FFT-clone F: ATGGATGCTCAAGACATTGAG
TC阳性PCR检测
Positive PCR detectionR: TTAAAAATGATAAAAATCATTG
TAAGTGGAGTTCAs6G-FFT F: TGGCTCTTTACGCACTCA 实时荧光定量
PCR分析
qRT-PCR analysisR: TCGCACTCGTCCTACCTC NtACT F: AATGATCGGAATGGAAGCTG R: TGGTACCACCACTGAGGACA 表 2 转基因烟草中As6G-FFT基因拷贝数
Table 2. Copy number of As6G-FFT in transgenic tobacco plants
转基因烟草
Transgenic tobaccoAs6G-FFT基因
As6G-FFT geneNtACT基因
NtACT gene浓度对数值的比值
Log value ratio of concentration拷贝数
Copy numberCt 熔解温度
Tm浓度对数值
Log value of concentrationCt 熔解温度
Tm浓度对数值
Log value of concentrationN1 19.14 83.81 2.55 20.60 80.13 2.22 1.15 1 N2 20.44 83.67 2.93 23.40 80.12 1.43 2.04 2 N3 20.81 83.70 3.03 20.78 80.10 2.17 1.40 1 N4 21.08 83.79 3.11 20.62 80.25 2.21 1.41 1 N5 21.66 83.73 3.28 23.57 80.23 1.38 2.37 2 N6 20.62 83.60 2.98 21.76 80.04 1.89 1.57 2 N7 19.72 83.61 2.72 21.53 80.23 1.96 1.39 1 N8 18.98 83.78 2.50 21.33 80.38 2.01 1.24 1 N9 23.51 83.75 3.82 23.82 80.19 1.31 2.91 3 N10 22.48 83.66 3.52 22.39 80.08 1.71 2.05 2 N11 22.46 83.79 3.51 21.78 80.21 1.89 1.86 2 N12 21.80 83.84 3.32 22.60 80.29 1.66 2.01 2 N13 22.76 83.69 3.60 22.75 80.12 1.61 2.23 2 N14 22.72 83.66 3.59 23.42 80.03 1.43 2.52 3 -
[1] 陈真真, 周国勤, 陈新宏, 等. 转华山新麦草果聚糖合成酶基因对烟草相关生理指标的影响 [J]. 湖北农业科学, 2020, 59(9):95−98.CHEN Z Z, ZHOU G Q, CHEN X H, et al. Effects of transfer of fructan synthase gene of Psathyrostachys huashanensis on physiological indexes of tobacco [J]. Hubei Agricultural Sciences, 2020, 59(9): 95−98.(in Chinese) [2] 许欢欢, 康健, 梁明祥. 植物果聚糖的代谢途径及其在植物抗逆中的功能研究进展 [J]. 植物学报, 2014, 49(2):209−220. doi: 10.3724/SP.J.1259.2014.00209XU H H, KANG J, LIANG M X. Research advances in the metabolism of fructan in plant stress resistance [J]. Chinese Bulletin of Botany, 2014, 49(2): 209−220.(in Chinese) doi: 10.3724/SP.J.1259.2014.00209 [3] FILIPENKO E A, FILIPENKO M L, DEINEKO E V, et al. Analysis of integration sites of T-DNA insertions in transgenic tobacco plants [J]. Cytology and Genetics, 2007, 41(4): 199−203. doi: 10.3103/S0095452707040019 [4] GORDANA M, MANSOUR K, MIRANDE N, et al. Gene silencing induced by hairpin or inverted repeated sense transgenes varies among promoters and cell types [J]. The New Phytologist, 2009, 184(4): 851−864. doi: 10.1111/j.1469-8137.2009.03011.x [5] LASSEUR B, LOTHIER J, DJOUMAD A, et al. Molecular and functional characterization of a cDNA encoding fructan: Fructan 6G-fructosyltransferase (6G-FFT)/fructan: Fructan 1-fructosyltransferase (1-FFT) from perennial ryegrass (Lolium perenne L. ) [J]. Journal of Experimental Botany, 2006, 57(11): 2719−2734. doi: 10.1093/jxb/erl034 [6] GITTE G, THOMAS D, MARIANNE F, et al. Improved fructan accumulation in perennial ryegrass transformed with the onion fructosyltransferase genes 1-SST and 6G-FFT [J]. Journal of Plant Physiology, 2008, 165(11): 1214−1225. doi: 10.1016/j.jplph.2007.06.019 [7] 何娜, 张园, 林春, 等. 芦笋果聚糖: 果聚糖6-果糖基转移酶基因Ao6G-FFT序列特征及表达模式研究 [J]. 植物生理学报, 2021, 57(4):929−938.HE N, ZHANG Y, LIN C, et al. Sequence characteristics and expression pattern of fructose 6G-fructose transferase gene Ao6G-FFT in Asparagus officinalis [J]. Plant Physiology Journal, 2021, 57(4): 929−938.(in Chinese) [8] 罗滨, 陈永康, 王莹. 植物外源基因拷贝数及插入位点的检测方法与技术 [J]. 河南师范大学学报(自然科学版), 2012, 40(6):111−116.LUO B, CHEN Y K, WANG Y. Methods and techniques for estimating the copy number and flanking sequences of exogenous gene in transgenic plants [J]. Journal of Henan Normal University(Natural Science Edition), 2012, 40(6): 111−116.(in Chinese) [9] 王育花, 赵森, 陈芬, 等. 利用实时荧光定量PCR法检测转基因水稻外源基因拷贝数的研究 [J]. 生命科学研究, 2007, 11(4):301−305. doi: 10.3969/j.issn.1007-7847.2007.04.004WANG Y H, ZHAO S, CHEN F, et al. Estimation of the copy number of exogenous gene in transgenic rice by real-time fluorescence quantitative PCR [J]. Life Science Research, 2007, 11(4): 301−305.(in Chinese) doi: 10.3969/j.issn.1007-7847.2007.04.004 [10] 裘劼人, 许颖, 喻富根. 利用SYBR Green实时定量PCR法检测转基因植物外源基因的拷贝数 [J]. 安徽农业科学, 2011, 39(21):12655−12657. doi: 10.3969/j.issn.0517-6611.2011.21.010QIU J R, XU Y, YU F G. Estimating the copy number of transgenes in transformed Arabidopsis by SYBR green real-time quantitative PCR [J]. Journal of Anhui Agricultural Sciences, 2011, 39(21): 12655−12657.(in Chinese) doi: 10.3969/j.issn.0517-6611.2011.21.010 [11] 苏慧慧, 李涛, 谢雯琦, 等. 基于实时荧光定量PCR对转基因樱桃番茄外源基因拷贝数的检测 [J]. 分子植物育种, 2015, 13(2):345−354.SU H H, LI T, XIE W Q, et al. Detecting exogenous gene copy numbers of exogenous gene in transgenic tomato based on fluorescent quantitative real-time PCR [J]. Molecular Plant Breeding, 2015, 13(2): 345−354.(in Chinese) [12] 田洁, 钟启文, 田萌, 等. 一种强酸水解-HPLC法检测大蒜果聚糖含量的方法: CN107941952A[P]. 2018-04-20. [13] 高洁铭. 菊芋块茎表皮花青素生物合成分子机理研究[D]. 西宁: 青海大学, 2020.GAO J M. Molecular mechanism of anthocyanin biosynthesis in tuber epidermis of Jerusalem artichoke[D]. Xining: Qinghai University, 2020. (in Chinese) [14] 王盛, 谢芝勋, 谢丽基, 等. 转基因烟草中外源基因实时荧光定量PCR检测方法的建立 [J]. 南方农业学报, 2015, 46(5):745−749. doi: 10.3969/j:issn.2095-1191.2015.5.745WANG S, XIE Z X, XIE L J, et al. Detection method of exogenous gene in transgenic tobacco by real-time fluorescence quantitative PCR [J]. Journal of Southern Agriculture, 2015, 46(5): 745−749.(in Chinese) doi: 10.3969/j:issn.2095-1191.2015.5.745 [15] 许兰珍, 何永睿, 雷天刚, 等. 转基因柑橘外源基因拷贝数的实时荧光定量PCR检测 [J]. 园艺学报, 2016, 43(6):1186−1194.XU L Z, HE Y R, LEI T G, et al. Identification of the copy number of exogenous gene in transgenic Citrus by quantitative real-time PCR [J]. Acta Horticulturae Sinica, 2016, 43(6): 1186−1194.(in Chinese) [16] 余婧, 邹颉, 付强, 等. 多重实时荧光定量PCR分析转基因烟草外源基因拷贝数 [J]. 中国烟草学报, 2017, 23(4):92−97.YU J, ZOU J, FU Q, et al. Detecting copy number of exogenous genes in transgenic tobacco by multiplex RT-PCR [J]. Acta Tabacaria Sinica, 2017, 23(4): 92−97.(in Chinese) [17] 余桂容, 张维, 杜文平, 等. 抗草甘膦转基因玉米外源基因ddPCR拷贝数分析 [J]. 西南农业学报, 2017, 30(8):1707−1712.YU G R, ZHANG W, DU W P, et al. Estimation of exogenous genes copy number of genetically modified glyphosate-resistant maize by droplet digital PCR [J]. Southwest China Journal of Agricultural Sciences, 2017, 30(8): 1707−1712.(in Chinese) [18] 王永, 兰青阔, 赵新, 等. 数字PCR在转基因水稻拷贝数鉴定中的应用 [J]. 生物技术通报, 2018, 34(3):53−58.WANG Y, LAN Q K, ZHAO X, et al. Estimation of the copy number of exogenous genes in genetically modified rice by droplet digital PCR [J]. Biotechnology Bulletin, 2018, 34(3): 53−58.(in Chinese) [19] 冀志庚, 高学军, 敖金霞, 等. SYBR Green实时定量PCR检测转基因大豆中外源基因拷贝数 [J]. 东北农业大学学报, 2011, 42(10):11−15.JI Z G, GAO X J, AO J X, et al. Establishment of SYBR Green-base quantitative real-time PCR assay for determining transgene copy number in transgenic soybean [J]. Journal of Northeast Agricultural University, 2011, 42(10): 11−15.(in Chinese) [20] 庄强, 钱程, 刘立. SYBR Green实时定量PCR检测外源基因拷贝数 [J]. 浙江理工大学学报, 2010, 27(1):125−129.ZHUANG Q, QIAN C, LIU L. Establishment of SYBR green-base quantitative real-time PCR assay for determining transgene copy number in genome [J]. Journal of Zhejiang Sci-Tech University, 2010, 27(1): 125−129.(in Chinese) [21] TAN B, LI D L, XU S X, et al. Highly efficient transformation of the GFP and MAC12.2 genes into precocious trifoliate orange (Poncirus trifoliata[L. ]Raf), a potential model genotype for functional genomics studies in Citrus [J]. Tree Genetics & Genomes, 2009, 5(3): 529−537. [22] WEN L, TAN B, GUO W W. Estimating transgene copy number in precocious trifoliate orange by TaqMan real-time PCR [J]. Plant Cell, Tissue and Organ Culture (PCTOC), 2012, 109(2): 363−371. doi: 10.1007/s11240-011-0101-x [23] 魏毅东, 罗曦, 吴方喜, 等. 利用qPCR法检测OsPIMT1转基因水稻中的外源片段拷贝数 [J]. 福建稻麦科技, 2017, 35(4):39−42. doi: 10.3969/j.issn.1008-9799.2017.04.013WEI Y D, LUO X, WU F X, et al. Determination of the copy number of exogenous fragment in Os PIMT1 transgenic rice by quantitative real-time PCR [J]. Fujian Science and Technology of Rice and Wheat, 2017, 35(4): 39−42.(in Chinese) doi: 10.3969/j.issn.1008-9799.2017.04.013 [24] 杜京尧, 尚飞, 王高华, 等. OsRhoGDI2过表达转基因水稻的筛选鉴定及外源基因拷贝数的初步分析 [J]. 江苏农业科学, 2019, 47(14):50−54.DU J Y, SHANG F, WANG G H, et al. Screening and identification ofOsRhoGDI2 overexpression transgenic rice and preliminary analysis of foreign gene copy number [J]. Jiangsu Agricultural Sciences, 2019, 47(14): 50−54.(in Chinese) -






 下载:
下载: