Interaction Identification between Magnaporthe oryzae Avirulence Effector Avr-PikD and Rice Protein OsDjA9
-
摘要:
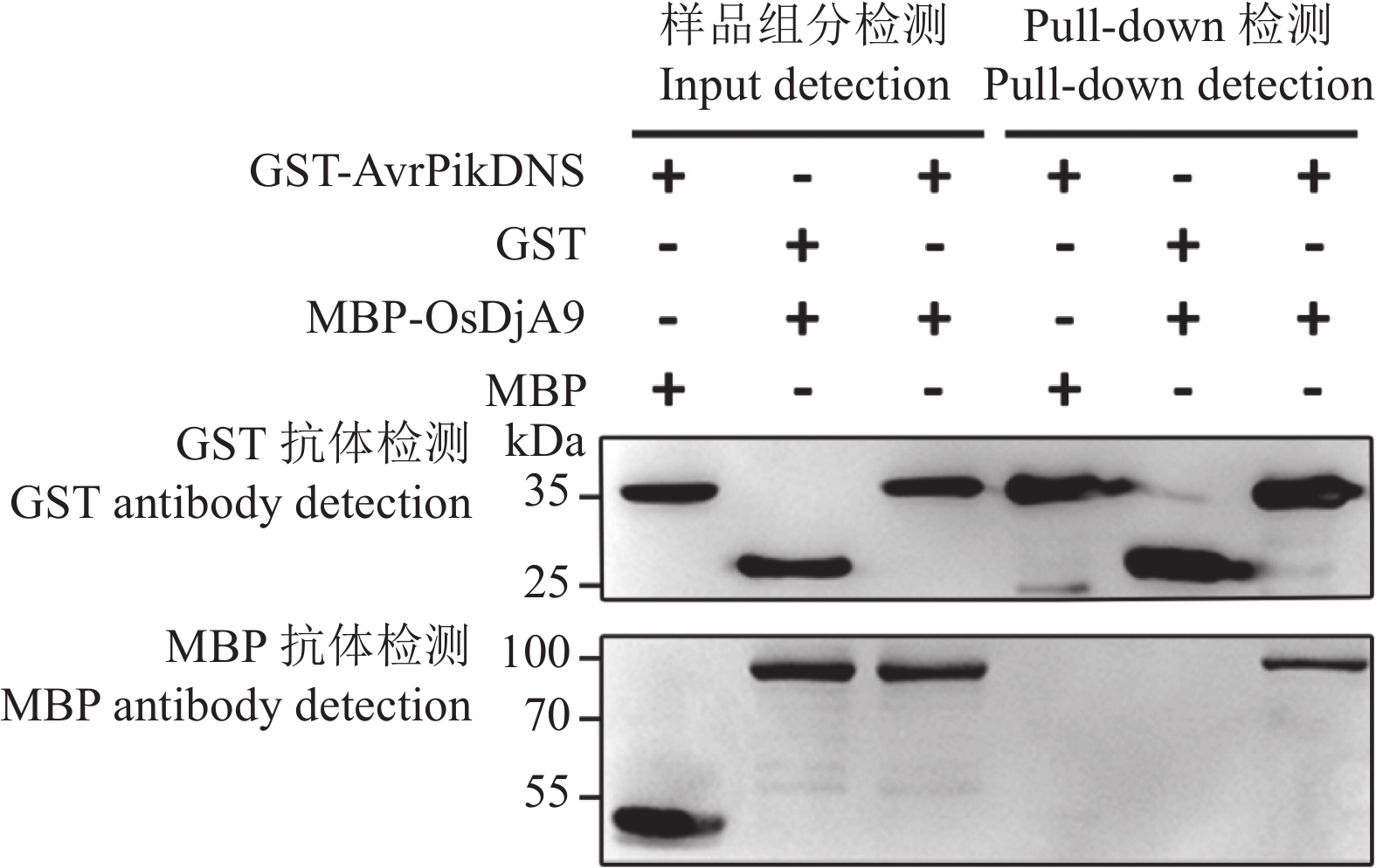
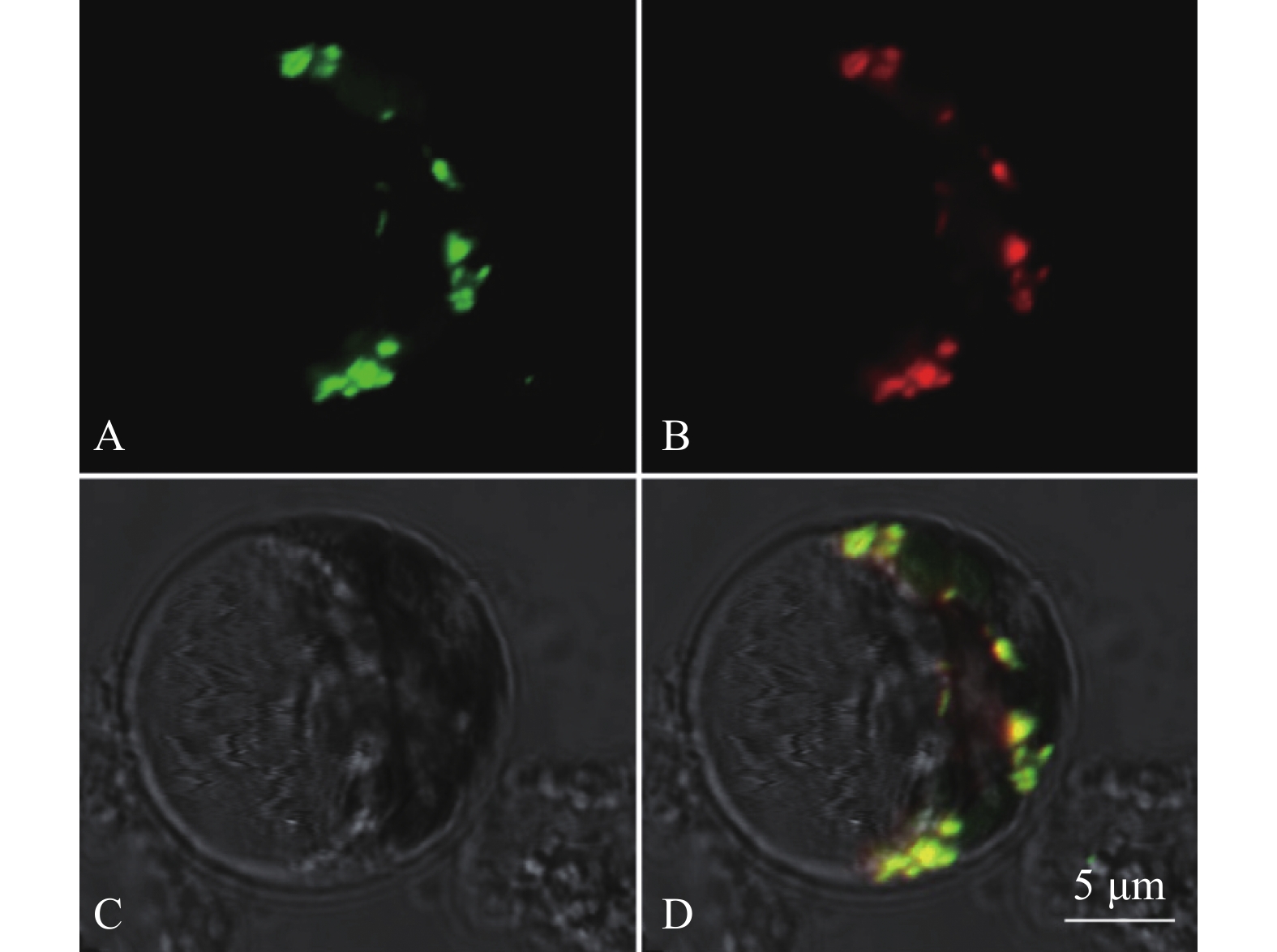
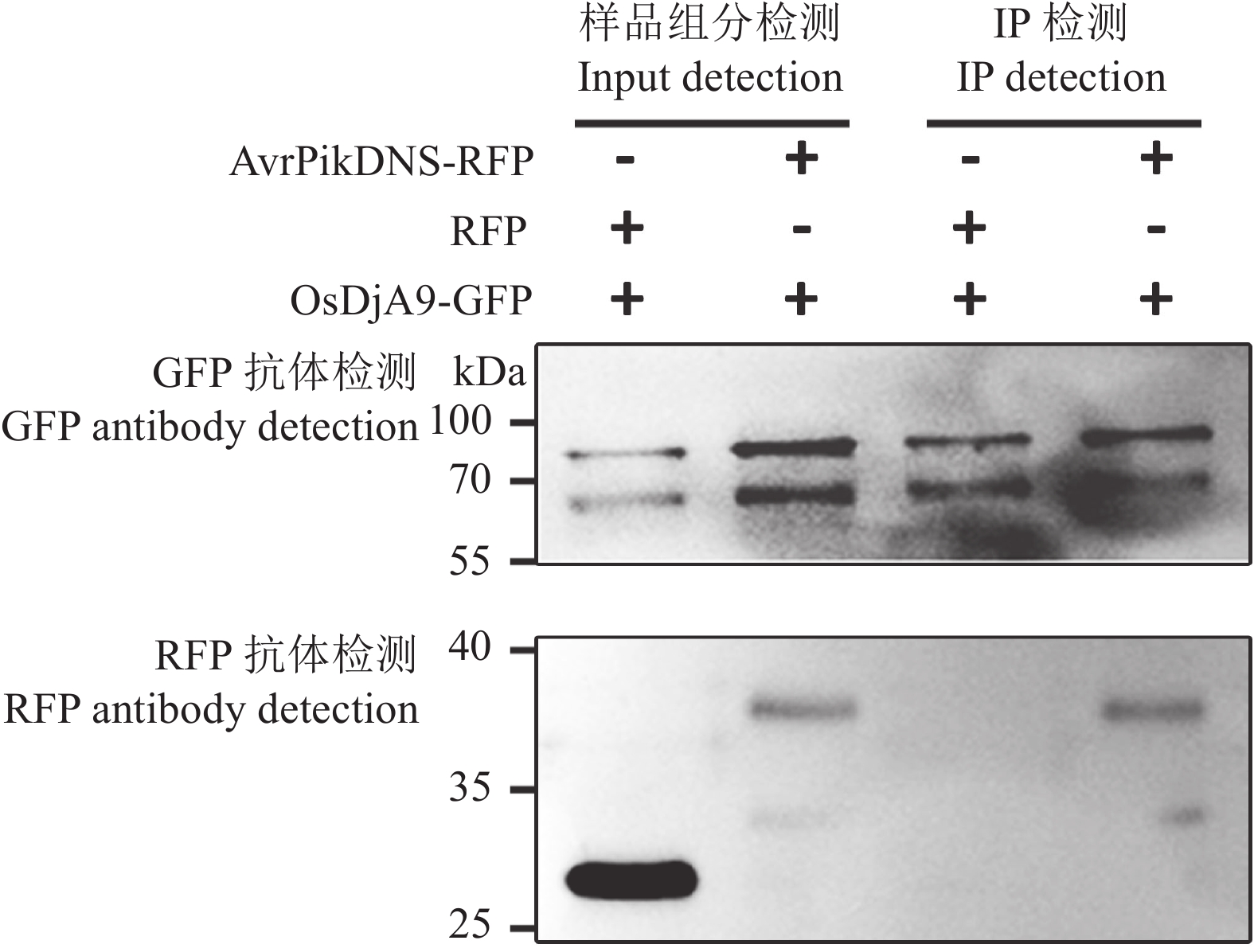
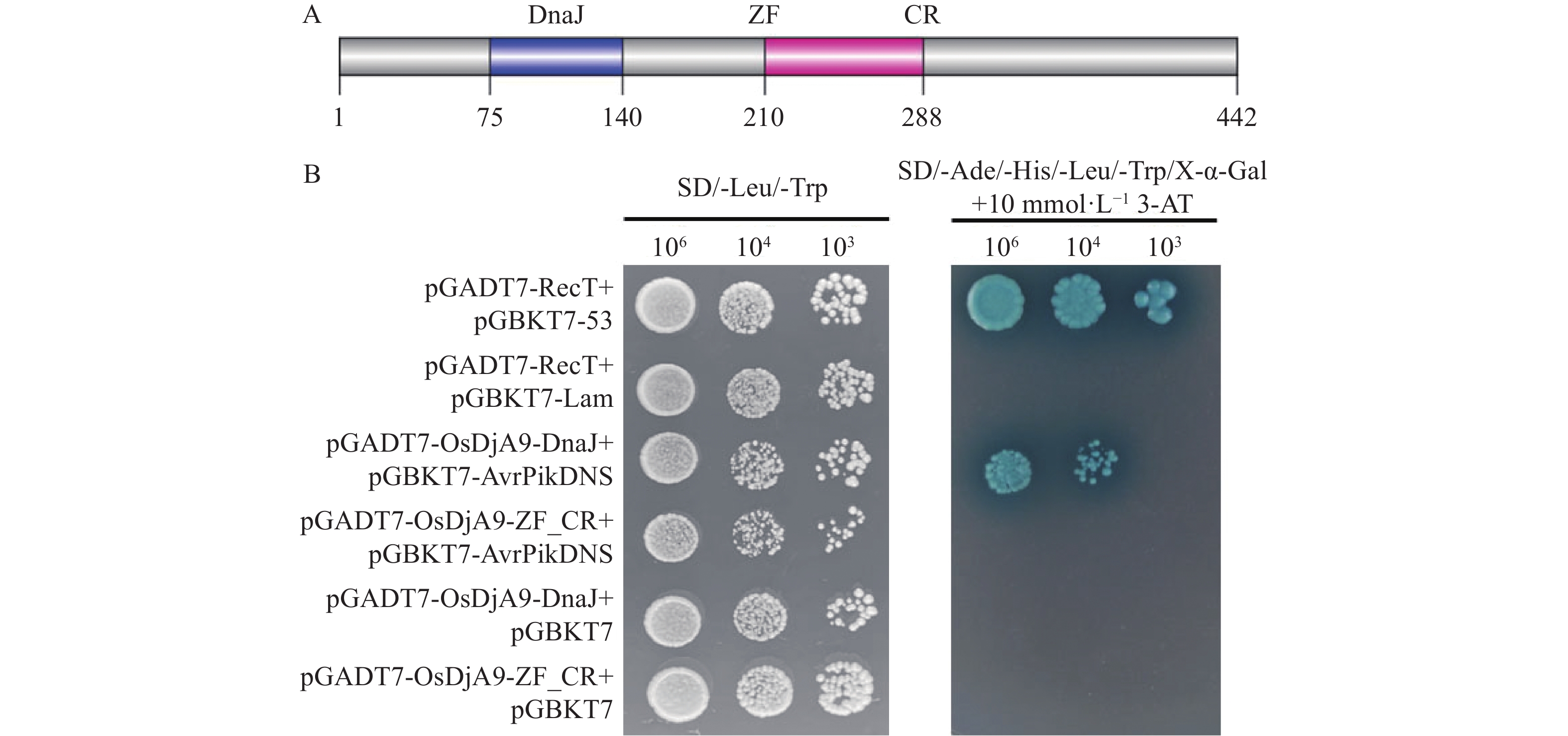
目的 对从水稻cDNA文库中筛选出的一个候选互作蛋白OsDjA9与Avr-PikD的互作关系进行鉴定,以期获得稻瘟病菌无毒效应因子Avr-PikD的水稻靶标。 方法 通过酵母双杂交、pull-down、Co-IP、荧光素酶互补成像试验及水稻原生质体中的共定位分析来验证Avr-PikD与OsDjA9的互作关系,并进一步借助酵母双杂交鉴定OsDjA9中参与互作的关键结构域。 结果 Avr-PikD在体外及体内条件下均能与OsDjA9发生相互作用,且OsDjA9所含DnaJ结构域是其与Avr-PikD互作所必需的。 结论 在稻瘟病菌侵染水稻的过程中,水稻OsDjA9蛋白是稻瘟病菌无毒效应因子Avr-PikD的一个作用靶标。 -
关键词:
- 稻瘟病菌 /
- 无毒效应因子Avr-PikD /
- 水稻 /
- OsDjA9 /
- 蛋白互作
Abstract:Objective To obtain the rice target of Magnaporthe oryzae avirulence effector Avr-PikD, the interaction between Avr-PikD and OsDjA9, one of the candidate interacting proteins screened out from a rice cDNA library, was identified. Method The interaction between Avr-PikD and OsDjA9 was verified by assays of yeast two-hybrid, pull-down, Co-IP, and luciferase complementation imaging along with co-localization analysis in the rice protoplasts. Specific domain in OsDjA9 involved in the interaction was determined by yeast two-hybrid assay. Result Avr-PikD interacts with OsDjA9 in vitro as well as in vivo. The DnaJ domain of OsDjA9 is essential for the interaction. Conclusion During the process of M. oryzae infecting rice, OsDjA9 is the target of the avirulence effector Avr-PikD secreted by the pathogen. -
Key words:
- Magnaporthe oryzae /
- avirulence effector Avr-PikD /
- rice /
- OsDjA9 /
- protein interaction
-
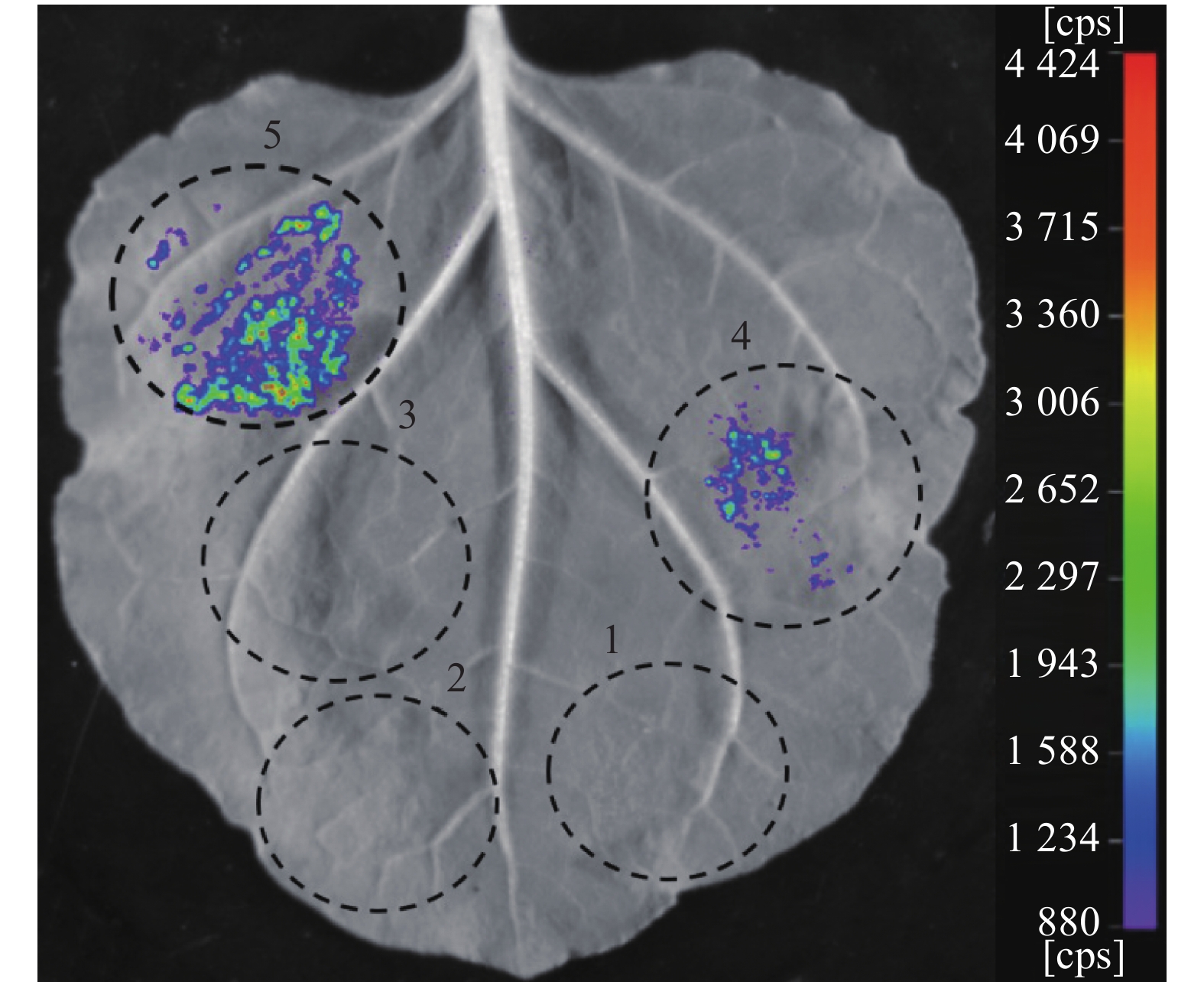
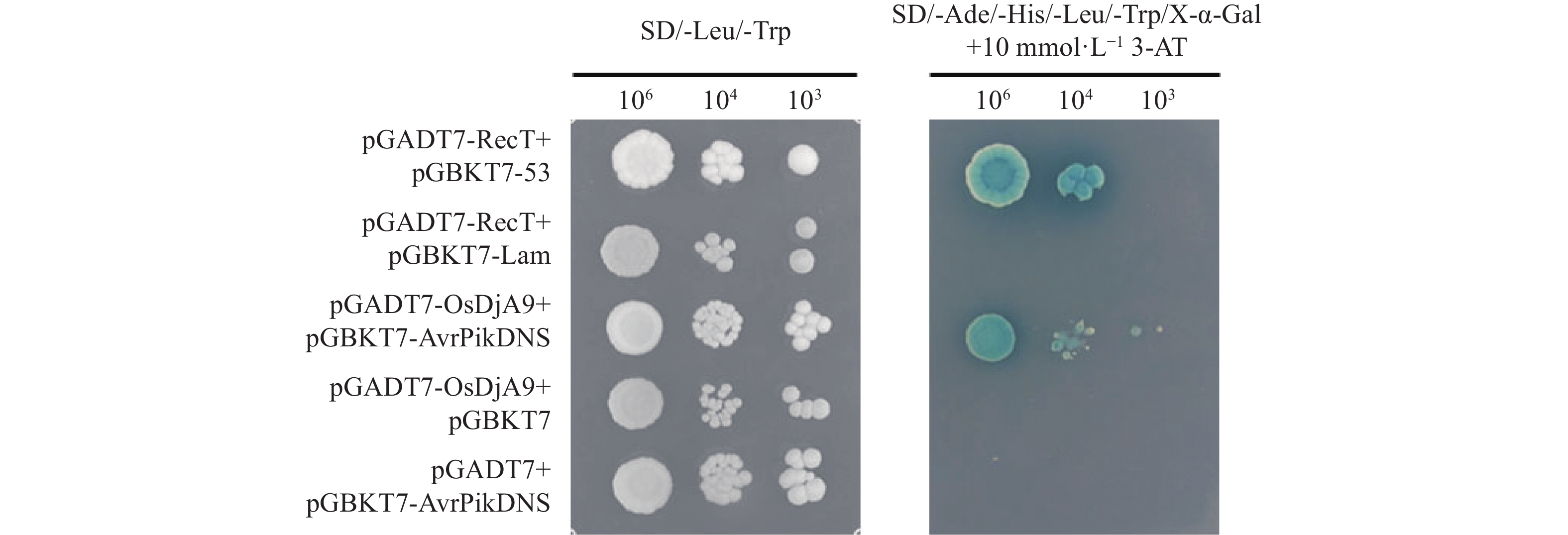
图 5 荧光素酶互补成像试验验证Avr-PikD与OsDjA9的互作关系
1,阴性对照组:pCAMBIA1300-NLuc与pCAMBIA1300-Cluc;2,阴性对照组:NLuc-AvrPikDNS与pCAMBIA1300-CLuc;3,阴性对照组:pCAMBIA1300-NLuc与CLuc-OsDjA9;4,试验组:NLuc-AvrPikDNS与CLuc-OsDjA9;5,阳性对照组:NLuc-AvrPiz-t与CLuc-APIP5。
Figure 5. Interaction between Avr-PikD and OsDjA9 verified by LCI assay
1, negative control: pCAMBIA1300-NLuc+pCAMBIA1300-Cluc; 2, negative control: NLuc-AvrPikDNS+pCAMBIA1300-CLuc; 3, negative control: pCAMBIA1300-NLuc+CLuc-OsDjA9; 4, test group: NLuc-AvrPikDNS+CLuc-OsDjA9; 5, positive control: NLuc-AvrPiz-t+CLuc-APIP5.
表 1 目前已知的稻瘟病菌无毒效应因子的水稻靶标
Table 1. Currently known rice targets for AVR effectors of M. oryzae
无毒效应
因子
Avirulence
effector水稻靶标
Rice target水稻靶标的功能注释
Functional annotation
of rice target参考文献
ReferenceAvrPiz-t APIP6 RING型E3泛素连接酶
RING-type E3 ligase[6] APIP10 RING型E3泛素连接酶
RING-type E3 ligase[7] APIP5 bZIP型转录因子
bZIP-type transcription factor[8] APIP12 核孔蛋白Nup98同源蛋白
Homolog of nucleoporin 98
(Nup98)[9] APIP7 (OsAKT1) K+通道蛋白
Potassium channel protein[10] APIP4 Bowman-Birk型胰蛋白酶抑制剂
Bowman-Birk-type trypsin inhibitor[11] Avr-Pii OsExo70-F2/F3 胞吐复合物亚基
Exocyst complex subunit[12] Os-NADP-ME2-3 NADP-苹果酸酶
NADP-malic enzyme[13] Avr-Pita OsCOX11 细胞色素c氧化酶组装蛋白
Cytochrome c oxidase assembly protein[14] 表 2 本研究所用引物序列
Table 2. Sequences of primers used in this study
引物名称
Primer name引物序列
Primer sequenceY2H-F/R TCCCCCCGGGTATGCGGCTCCCCGGCGACGC/CCGCTCGAGCTATCCCGATGCTCCTGCTGCCTTT GST-F/R CGGGATCCATGGAAACGGGCAACAAA/
CGGAATTCTTAAAAGCCGGGCCTTTTMBP-F/R GCTCTAGAATGCGGCTCCCCGGCGACGC/
ACGCGTCGACCTATCCCGATGCTCCTGCTGCCTTTGFP-F/R CGGGATCCATGCGGCTCCCCGGCGAC/GGGGTACCTCCCGATGCTCCTGCTGC RFP-F/R CGGGATCCATGGAAACGGGCAACAAATAT/
GGGGTACCAAAGCCGGGCCTTTTTTTCCCNLuc-F/R CGAGCTCGGTACCCGGGATCCATGGAAACGGGCAACAAATATATA/
CGCGTACGAGATCTGGTCGACAAAGCCGGGCCTTTTTTTCCLuc-F/R GGCGGTACCCGGGATCCAATGCGGCTCCCCGGCGACGCT/
GAAAGCTCTGCAGGTCGACCTATCCCGATGCTCCTGCTGCDnaJ-F/R GGAATTCCATATGCACGGGACGAGGCCG/
CGGGATCCCTAAAGTGCAACCTTGGCZF_CR-F/R GGAATTCCATATGGAAATATCATTTATGGAAGC/
CGGGATCCCTAATCCGTACCAGGCAC -
[1] TALBOT N J. On the trail of a cereal killer: Exploring the biology of Magnaporthe grisea [J]. Annual Review of Microbiology, 2003, 57: 177−202. doi: 10.1146/annurev.micro.57.030502.090957 [2] SKAMNIOTI P, GURR S J. Against the grain: Safeguarding rice from rice blast disease [J]. Trends in Biotechnology, 2009, 27(3): 141−150. doi: 10.1016/j.tibtech.2008.12.002 [3] MENTLAK T A, KOMBRINK A, SHINYA T, et al. Effector-mediated suppression of chitin-triggered immunity by Magnaporthe oryzae is necessary for rice blast disease [J]. The Plant Cell, 2012, 24(1): 322−335. doi: 10.1105/tpc.111.092957 [4] IRIEDA H, INOUE Y, MORI M, et al. Conserved fungal effector suppresses PAMP-triggered immunity by targeting plant immune kinases [J]. PNAS, 2019, 116(2): 496−505. doi: 10.1073/pnas.1807297116 [5] WANG B H, EBBOLE D J, WANG Z H. The arms race between Magnaporthe oryzae and rice: Diversity and interaction of Avr and R genes [J]. Journal of Integrative Agriculture, 2017, 16(12): 2746−2760. doi: 10.1016/S2095-3119(17)61746-5 [6] PARK C H, CHEN S B, SHIRSEKAR G, et al. The Magnaporthe oryzae effector AvrPiz-t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen-associated molecular pattern-triggered immunity in rice [J]. The Plant Cell, 2012, 24(11): 4748−4762. doi: 10.1105/tpc.112.105429 [7] PARK C H, SHIRSEKAR G, BELLIZZI M, et al. The E3 ligase APIP10 connects the effector AvrPiz-t to the NLR receptor Piz-t in rice [J]. PLoS Pathogens, 2016, 12(3): e1005529. doi: 10.1371/journal.ppat.1005529 [8] WANG R Y, NING Y S, SHI X T, et al. Immunity to rice blast disease by suppression of effector-triggered necrosis [J]. Current Biology, 2016, 26(18): 2399−2411. doi: 10.1016/j.cub.2016.06.072 [9] TANG M Z, NING Y S, SHU X L, et al. The Nup98 homolog APIP12 targeted by the effector AvrPiz-t is involved in rice basal resistance against Magnaporthe oryzae [J]. Rice (N Y), 2017, 10(1): 5−15. doi: 10.1186/s12284-017-0144-7 [10] SHI X T, LONG Y, HE F, et al. The fungal pathogen Magnaporthe oryzae suppresses innate immunity by modulating a host potassium channel [J]. PLoS Pathogens, 2018, 14(1): e1006878. doi: 10.1371/journal.ppat.1006878 [11] ZHANG C Y, FANG H, SHI X T, et al. A fungal effector and a rice NLR protein have antagonistic effects on a Bowman-Birk trypsin inhibitor [J]. Plant Biotechnology Journal, 2020, 18(11): 2354−2363. doi: 10.1111/pbi.13400 [12] FUJISAKI K, ABE Y, ITO A, et al. Rice Exo70 interacts with a fungal effector, AVR-Pii, and is required for AVR-Pii-triggered immunity [J]. The Plant Journal, 2015, 83(5): 875−887. doi: 10.1111/tpj.12934 [13] SINGH R, DANGOL S, CHEN Y F, et al. Magnaporthe oryzae effector AVR-pii helps to establish compatibility by inhibition of the rice NADP-malic enzyme resulting in disruption of oxidative burst and host innate immunity [J]. Molecules and Cells, 2016, 39(5): 426−438. doi: 10.14348/molcells.2016.0094 [14] HAN J L, WANG X Y, WANG F P, et al. The fungal effector avr-pita suppresses innate immunity by increasing COX activity in rice mitochondria [J]. Rice (New York, N Y ), 2021, 14(1): 12. [15] ZHAI K R, LIANG D, LI H L, et al. NLRs guard metabolism to coordinate pattern- and effector-triggered immunity [J]. Nature, 2022, 601(7892): 245−251. doi: 10.1038/s41586-021-04219-2 [16] KANZAKI H, YOSHIDA K, SAITOH H, et al. Arms race co-evolution of Magnaporthe oryzae AVR-Pik and rice Pik genes driven by their physical interactions [J]. The Plant Journal, 2012, 72(6): 894−907. doi: 10.1111/j.1365-313X.2012.05110.x [17] WU W H, WANG L, ZHANG S, et al. Stepwise arms race between AvrPik and Pik alleles in the rice blast pathosystem [J]. Molecular Plant Microbe Interactions, 2014, 27(8): 759−769. doi: 10.1094/MPMI-02-14-0046-R [18] ZHANG Y, SU J B, DUAN S, et al. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes [J]. Plant Methods, 2011, 7(1): 30. doi: 10.1186/1746-4811-7-30 [19] WANG G D, CAI G H, KONG F Y, et al. Overexpression of tomato chloroplast-targeted dnaj protein enhances tolerance to drought stress and resistance to Pseudomonas solanacearum in transgenic tobacco [J]. Plant Physiology and Biochemistry, 2014, 82: 95−104. doi: 10.1016/j.plaphy.2014.05.011 [20] ZHONG X H, YANG J X, SHI Y L, et al. The DnaJ protein OsDjA6 negatively regulates rice innate immunity to the blast fungus Magnaporthe oryzae [J]. Molecular Plant Pathology, 2018, 19(3): 607−614. doi: 10.1111/mpp.12546 [21] FENG H J, LI C, ZHOU J L, et al. A cotton WAKL protein interacted with a DnaJ protein and was involved in defense against Verticillium dahliae [J]. International Journal of Biological Macromolecules, 2021, 167: 633−643. doi: 10.1016/j.ijbiomac.2020.11.191 [22] CHEN S B, SONGKUMARN P, VENU R C, et al. Identification and characterization of in planta-expressed secreted effector proteins from Magnaporthe oryzae that induce cell death in rice [J]. Molecular Plant Microbe Interactions, 2013, 26(2): 191−202. doi: 10.1094/MPMI-05-12-0117-R [23] XU G J, ZHONG X H, SHI Y L, et al. A fungal effector targets a heat shock-dynamin protein complex to modulate mitochondrial dynamics and reduce plant immunity [J]. Science Advances, 2020, 6(48): eabb7719. doi: 10.1126/sciadv.abb7719 -
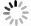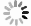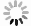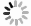






 下载:
下载: