Effects of Melatonin on Tomato Polyamine Metabolism and Cold Tolerance Gene Expression under Low Temperature Stress
-
摘要:
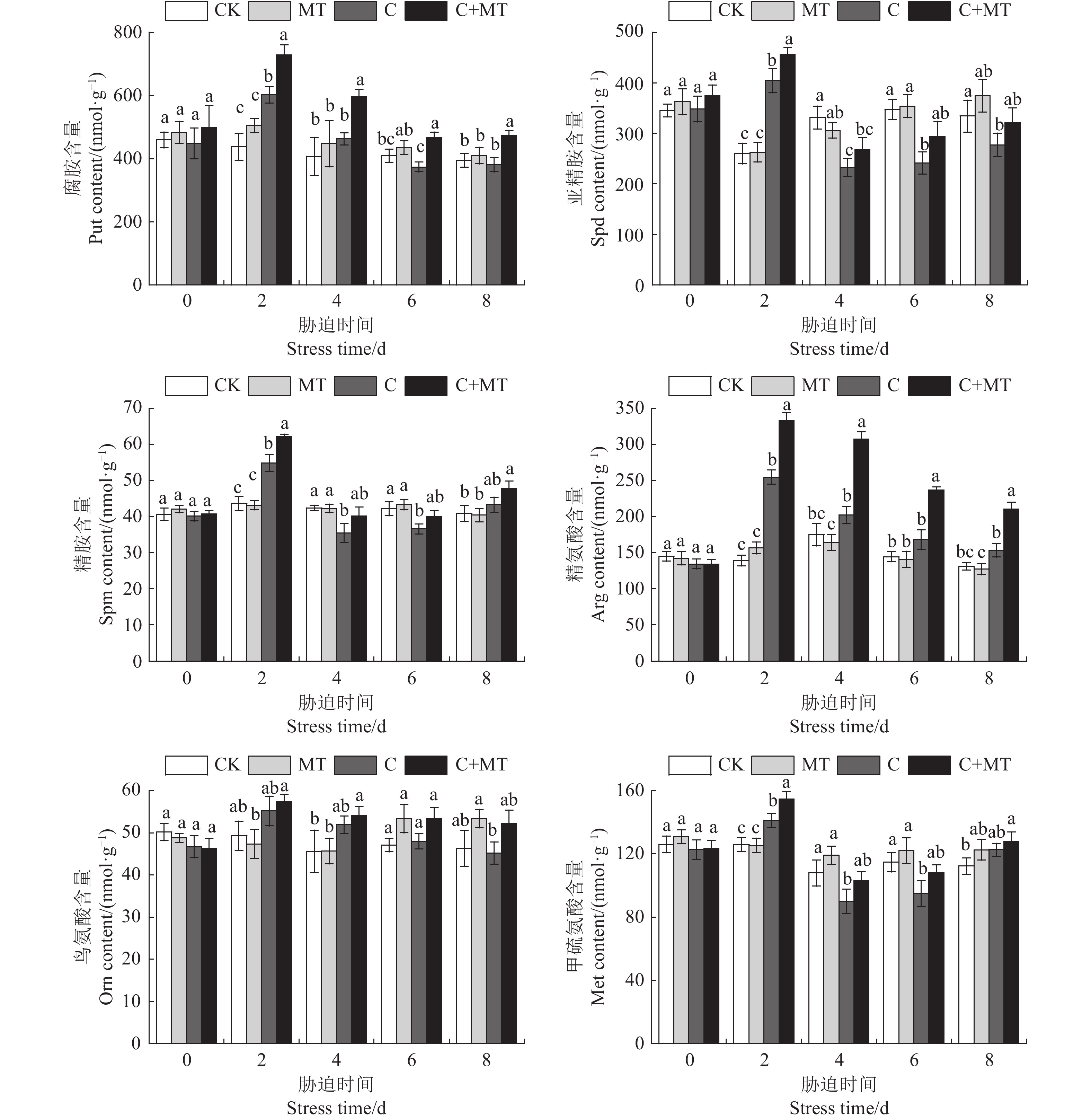
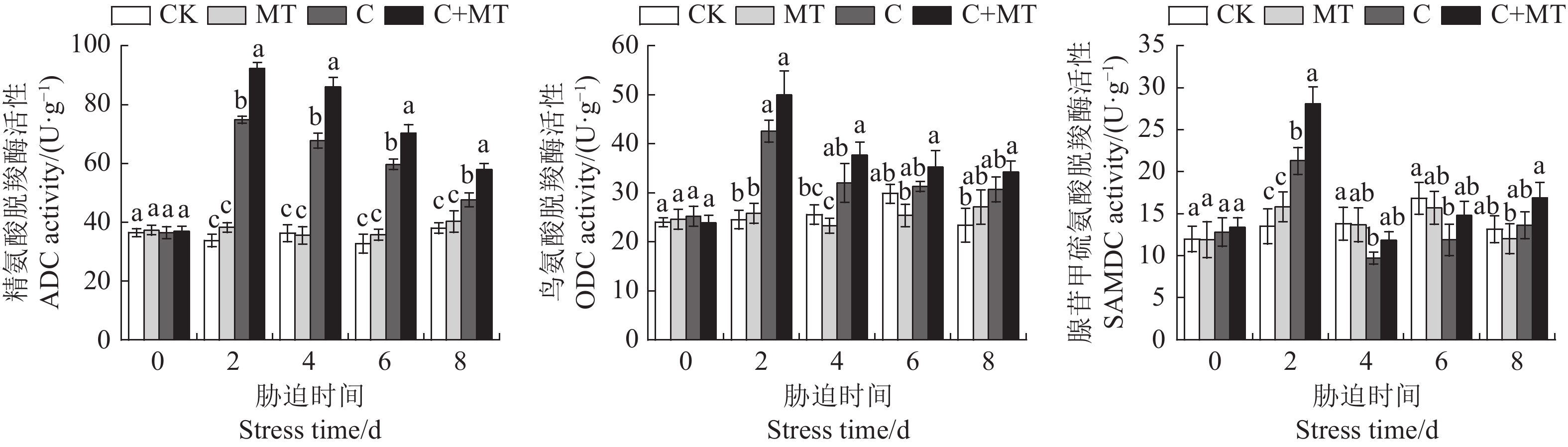
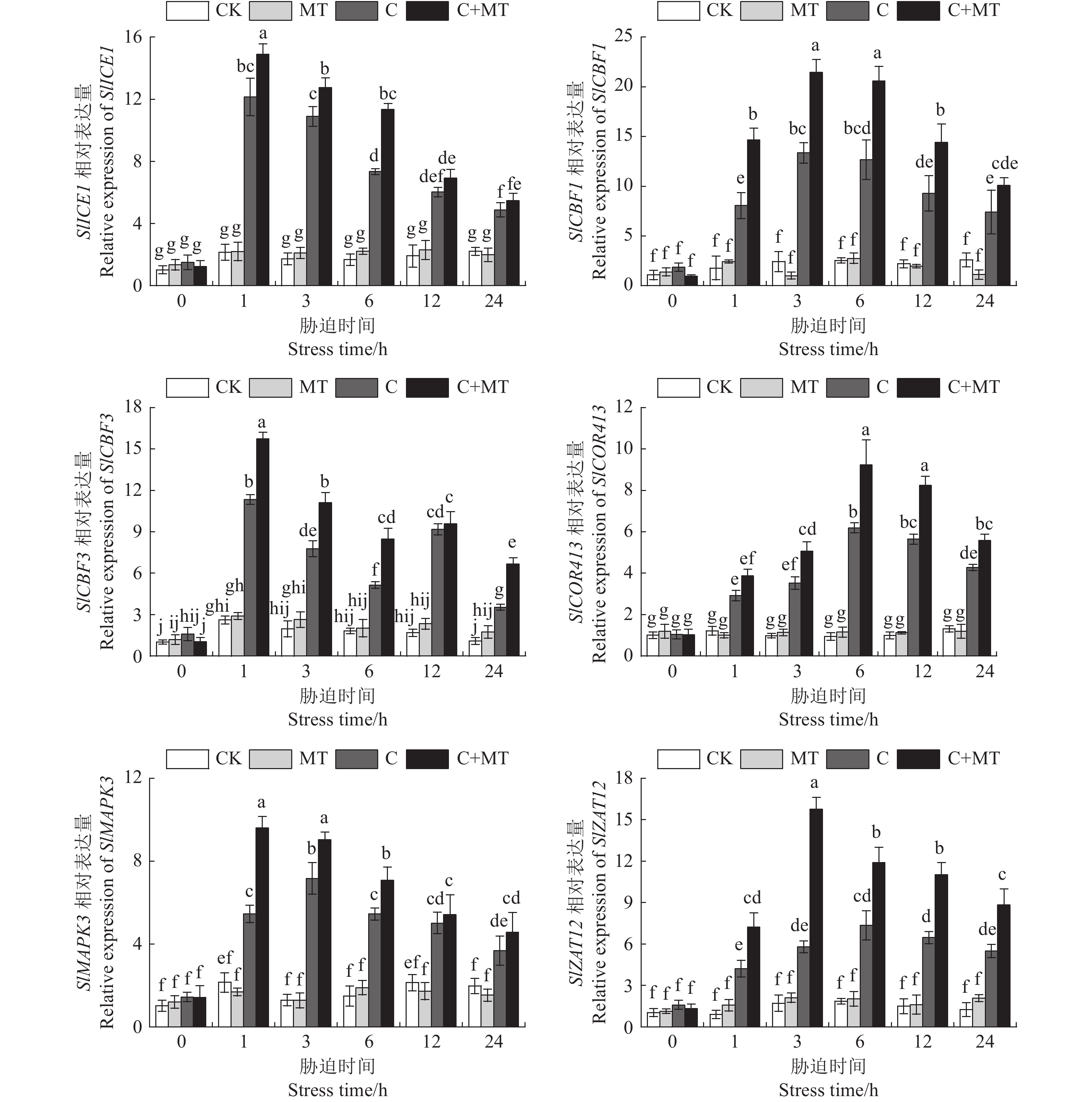
目的 探讨低温胁迫下褪黑素如何调节番茄幼苗多胺代谢,以及对番茄耐冷基因表达的影响,揭示褪黑素缓解番茄低温胁迫的机制。 方法 以番茄东农708为试验材料,采用叶片喷施的方法,研究不同浓度(50、100、200、300 µmol·L−1)褪黑素对番茄幼苗生长、多胺代谢以及耐冷基因表达的影响;并通过腐胺(Put)合成抑制剂与褪黑素联合预处理进行验证,分析抑制剂对低温胁迫下褪黑素诱导番茄多胺含量和抗氧化酶活性的影响。 结果 褪黑素预处理缓解了低温胁迫对番茄幼苗生长的抑制,显著提高了番茄株高、鲜/干质量及低温处理各时段番茄叶片中Put含量,而亚精胺(Spd)和精胺(Spm)含量仅在低温处理第2天显著增加,精氨酸(Arg)含量和精氨酸脱羧酶(ADC)活性及ADC合成相关基因表达水平也显著高于单独低温处理,Put分解酶二胺氧化酶(DAO)活性与基因表达水平显著降低,验证试验发现低温胁迫下Put的精氨酸途径抑制剂显著降低了褪黑素诱导的Put积累和抗氧化酶活性,阻碍了褪黑素对细胞膜的保护作用。同时,褪黑素提高了低温胁迫下番茄叶片内与Put相关的耐冷基因表达水平。 结论 褪黑素可正向调节精氨酸介导的Put合成途径并抑制Put分解来增加Put的积累,褪黑素通过精氨酸介导的Put积累提高番茄的抗氧化防御和耐冷基因的表达,从而增强番茄幼苗对低温胁迫的耐受性。 Abstract:Objective Polyamine metabolism and expression of cold tolerance genes associated with the mechanism of melatonin in alleviating low temperature stress in tomato were investigated. Method Solutions of melatonin in the concentrations of 50, 100, 200, and 300 µmol·L−1 were sprayed on the leaves of Dongnong 708 tomato plants (Solanum lycopersicum). Under low temperature, optimal concentration of melatonin for the stress alleviation was determined according to the malondialdehyde content and electrolyte leakage rate in the leaves. Effects of the treatment on the growth, polyamine metabolism, and cold tolerance gene expression, as well as those of putrescine (Put) synthesis inhibitor and melatonin pretreatment on polyamine content and antioxidant characteristics, of the plants were determined. Result Pretreating the tomato plans with melatonin prior to low temperature exposure lessened the seedling growth retardation induced by the stress. The plant height and fresh/dry weights as well as the Put in leaf at all stages of the stress imposition were significantly increased. The contents of spermidine (Spd) and spermine (Spm) rose significantly on the 2nd day after low temperature treatment before tapering off. The arginine (Arg) content, arginine decarboxylase (ADC) activity, and ADC synthesis-related gene expressions became significantly higher with the melatonin pretreatment, while the Put catabolic enzyme diamine oxidase (DAO) activity and gene expression significantly declined. A challenge test confirmed that, under low temperature stress, the Put synthesis inhibitor in the arginine pathway significantly reduced the melatonin-induced enhancements on Put accumulation and antioxidant enzyme activity, which would otherwise provide a protective effect on the cell membrane. Additionally, the expression of cold tolerance genes related to Put in tomato leaves was upregulated with the presence of melatonin. Conclusion Melatonin positively regulated the accumulation, but inhibited the degradation, of Put mediated by arginine. The arginine mediated Put synthesis pathway played an important role in the antioxidant defense of the tomato plants improved by melatonin. In addition, melatonin might also increase the expression of cold tolerance genes by way of promoting the Put synthesis to further boost the cold tolerance of tomato seedlings. -
Key words:
- Tomatos /
- melatonin /
- low temperature stress /
- polyamine metabolism /
- cold tolerance gene
-
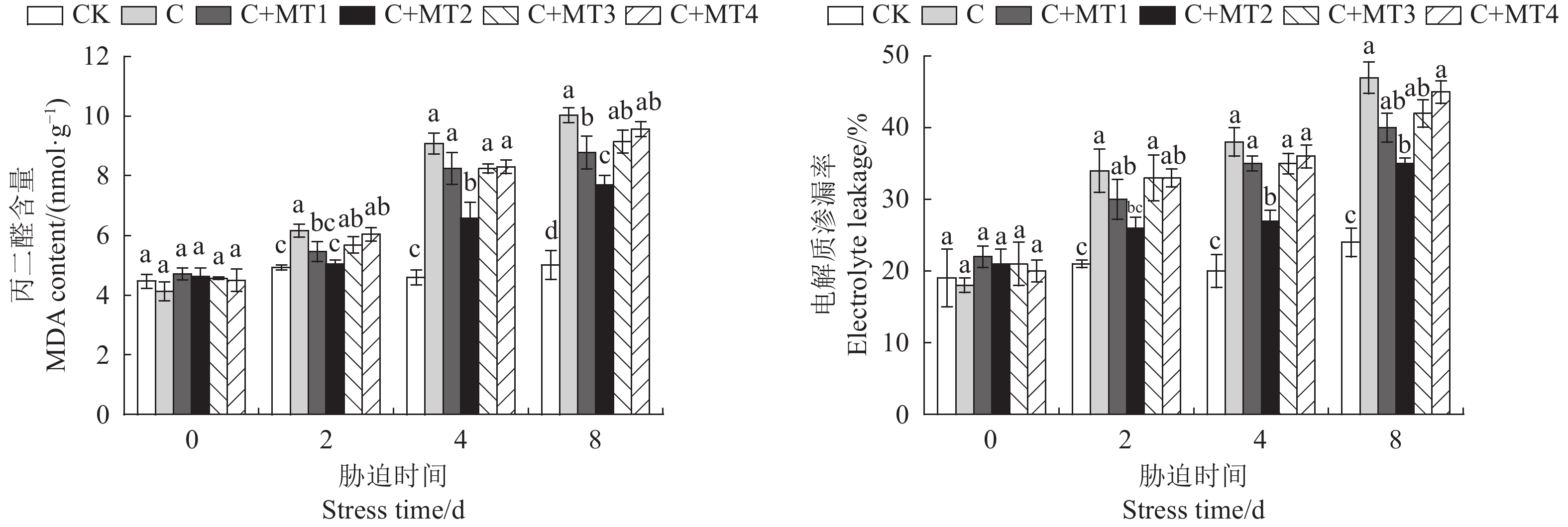
图 1 褪黑素对低温胁迫下番茄幼苗叶片MDA含量和电解质渗漏率的影响
CK:常温对照处理;C:低温处理;C+MT1-4分别为:50、100、200、300 μmol·L−1褪黑素预处理后进行低温处理。柱子上方不同小写字母表示处理间差异显著(P<0.05)。
Figure 1. Effects of melatonin on MDA content and electrolyte leakage in tomato leaves under low temperature stress
CK: Control under normal temperature; C: Cold treatment; C+MT1-4: 50, 100, 200, and 300 μmol·L−1 melatonin pretreatments, respectively, prior to low temperature exposure. Data with different lowercase letters on top of column indicate significant difference at p<0.05. Same for below.
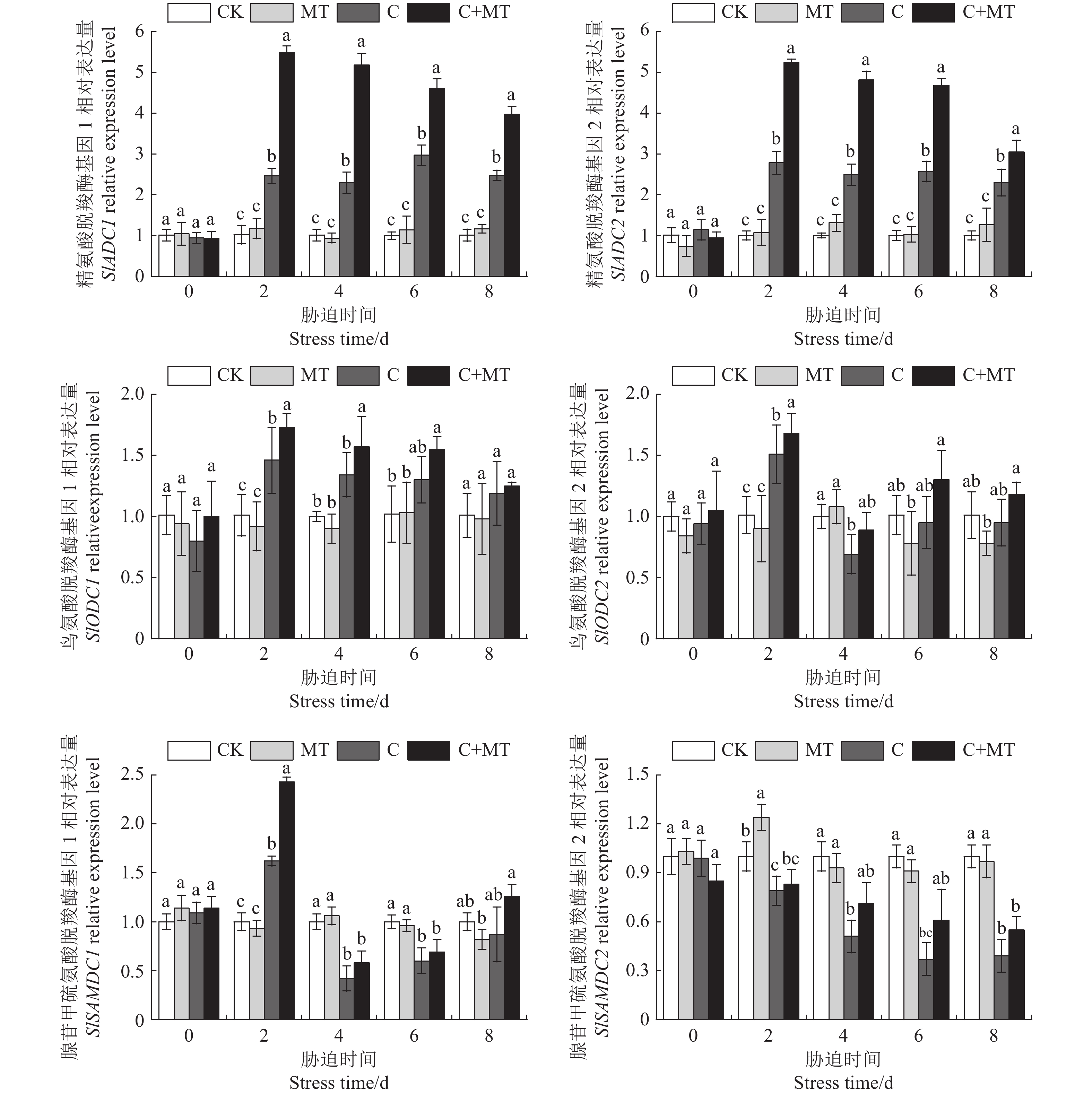
图 6 腐胺合成抑制剂对褪黑素诱导的番茄叶片多胺含量的影响
CK:常温处理;C:低温处理;C+MT:100 μmol·L−1褪黑素预处理后进行低温处理;C+D-Arg+MT:先用D-Arg预处理,然后用褪黑素处理,再进行低温处理;C+DFMO+MT:先用DFMO预处理,然后用褪黑素处理,再进行低温处理。图7、8同。
Figure 6. Effect of Put synthesis inhibitor on polyamine content of tomato leaves induced by melatonin
CK:Normal temperature control; C:Cold treatment; C+MT: 100 μmol·L−1 melatonin pretreatment and then cold treatment; C+D-Arg+MT: Pre-treated with D-Arg first, then treated with melatonin, and then treated at low temperature; C+DFMO+MT: Pre-treated with DFMO first, then treated with melatonin, and then treated at low temperature. Same for Fig 7, 8.
表 1 多胺合成前体物质检测流动相梯度洗脱程序
Table 1. Mobile phase elution for detection of polyamine synthesis precursors
时间
Time/minA/% B/% 流速
Elution velocity/(mL·min−1)0 100 0 1 4 96 4 1 13 85 10 1 25 60 40 1 30 60 40 1 表 2 qRT-PCR引物序列
Table 2. Primer sequences used in qRT-PCR
基因
Gene上游引物(5′-3′)
Forward primer下游引物(5′-3′)
Reverse primerSlADC1 CATCCAGTGATTTGCAGCGA GTAAACCACCCGAAGATGGC SlADC2 GTCGGATATGGCCTTCAGGA CTTCACGTTCTTCCGGTCAC SlODC1 GCTCAACTCGGAATGCCAAA CTCAGGGAAGTCGTGGAAGT SlODC2 GGAGCATTGCCGGAAGAAAT TCGCTTGGCGATAAATGGTG SlSAMDC1 CCGAGTCTAGCCTCTTCGTT AAATGAAGCTCCCACGGGTA SlSAMDC2 AAACTCTTATGGCCCTGGCT AGGCTGGACTCTGAAAGGAC SlDAO ATGATAGCCGCGCTAGACTT CCCTACTCCCAAGTTACCA SlPAO CCTCTGTGATCATCGTCGGA CTACTCCGCCGAATTCCTCT SlICE1 TGGAAGGAAAAGCGGTGAAC AACACATCCAACACAAACCC SCBF1 AGTCGGAGGAAGAAGAATCAGTG TCCCATTTCAGTACATTGAGGTG SlCBF3 GGCAATTTCATCTGAGTTGTCTG TTGATCTTCTGTCCATCCTCTCC SlCOR413 ATTGATGTAAGTGGAGAGAT AACTGCAGTAGGAGCTTGT SlMAPK3 AGCATTAGCTCATCCCTACCTC GCTCTTCTCCTATCCCTTGTTG SlZAT12 GCCATCGAACGAGTCATAAATC CCTGACCCATAGAAAACTCCAT Actin TGGTCGGAATGGGACAGAAG CTCAGTCAGGAGAACAGGGT 表 3 褪黑素对低温胁迫下番茄幼苗生长的影响
Table 3. Effects of melatonin spray on growth of tomato seedlings under low temperature stress
处理
Treatment株高
Plant height/cm地上部鲜质量
Shoot fresh matter/g地上部干质重
Shoot dry matter/g地下部鲜质量
Root fresh matter/g地下部干质量
Root dry matter/g全株鲜质量
Whole plant fresh matter/g全株干质量
Whole plant dry matter/gCK 16.49±0.32 a 7.15±0.15 a 0.91±0.03 a 2.50±0.16 a 0.45±0.02 a 9.65±0.30 a 1.36±0.05 a MT 16.54±0.45 a 7.41±0.35 a 0.90±0.03 a 2.43±0.24 a 0.47±0.02 a 9.84±0.53 a 1.38±0.01 a C 13.89±0.36 c 4.73±0.16 c 0.68±0.06 c 1.34±0.18 c 0.33±0.01 c 6.07±0.19 c 1.01±0.06 c C+MT 15.12±0.28 b 5.82±0.38 b 0.80±0.03 b 1.89±0.10 b 0.39±0.01 b 7.71±0.48 b 1.19±0.04 b CK:常温处理;MT:常温下100 µmol·L−1褪黑素处理;C:低温处理;C+MT:100 µmol·L−1褪黑素预处理后进行低温处理。表中不同小写字母表示处理间差异显著(P<0.05)。下同。
CK: Control under normal temperature; MT: 100 µmol·L−1 melatonin treatment at normal temperature; C: Cold treatment; C+MT: 100 µmol·L−1 melatonin pretreatment prior to low temperature exposure. Data with different lowercase letters on top of column indicate significant difference at P<0.05. Same for below. -
[1] LIU H, OUYANG B, ZHANG J H, et al. Differential modulation of photosynthesis, signaling, and transcriptional regulation between tolerant and sensitive tomato genotypes under cold stress [J]. PLoS One, 2012, 7(11): e50785. doi: 10.1371/journal.pone.0050785 [2] LIU Y F, QI M F, LI T L. Photosynthesis, photoinhibition, and antioxidant system in tomato leaves stressed by low night temperature and their subsequent recovery [J]. Plant Science, 2012, 196: 8−17. doi: 10.1016/j.plantsci.2012.07.005 [3] SHI H T, JIANG C, YE T T, et al. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass[Cynodon dactylon (L). Pers. ]by exogenous melatonin [J]. Journal of Experimental Botany, 2014, 66(3): 681−694. [4] GONG X Q, SHI S T, DOU F F, et al. Exogenous melatonin alleviates alkaline stress in Malus hupehensis rehd. by regulating the biosynthesis of polyamines [J]. Molecules (Basel, Switzerland), 2017, 22(9): 1542. doi: 10.3390/molecules22091542 [5] 张贵友, 李萍, 戴尧仁. 低温胁迫下褪黑激素对烟草悬浮细胞精氨酸脱羧酶活性的影响 [J]. 植物学通报, 2005, 40(5):555−559.ZHANG G Y, LI P, DAI Y R. Arginine decarboxylase activity is increased in tobacco (Nicotiana tabacum) suspension cells by exogenous melatonin during cold stress [J]. Chinese Bulletin of Botany, 2005, 40(5): 555−559.(in Chinese) [6] KE Q B, YE J, WANG B M, et al. Melatonin mitigates salt stress in wheat seedlings by modulating polyamine metabolism [J]. Frontiers in Plant Science, 2018, 9: 914. doi: 10.3389/fpls.2018.00914 [7] BOSE S K, HOWLADER P. Melatonin plays multifunctional role in horticultural crops against environmental stresses: A review [J]. Environmental and Experimental Botany, 2020, 176: 104063. doi: 10.1016/j.envexpbot.2020.104063 [8] SHI H T, CHAN Z L. The cysteine2/histidine2-type transcription factor zinc finger of Arabidopsis thaliana 6-activated c-repeat-binding factor pathway is essential for melatonin-mediated freezing stress resistance in Arabidopsis [J]. Journal of Pineal Research, 2014, 57(2): 185−191. doi: 10.1111/jpi.12155 [9] BAJWA V S, SHUKLA M R, SHERIF S M, et al. Role of melatonin in alleviating cold stress in Arabidopsis thaliana [J]. Journal of Pineal Research, 2014, 56(3): 238−245. doi: 10.1111/jpi.12115 [10] LI H, CHANG J J, ZHENG J X, et al. Local melatonin application induces cold tolerance in distant organs of Citrullus lanatus L. via long distance transport [J]. Scientific Reports, 2017, 7: 40858. doi: 10.1038/srep40858 [11] LEI X Y, ZHU R Y, ZHANG G Y, et al. Attenuation of cold-induced apoptosis by exogenous melatonin in carrot suspension cells: The possible involvement of polyamines [J]. Journal of Pineal Research, 2004, 36(2): 126−131. doi: 10.1046/j.1600-079X.2003.00106.x [12] LI H, HE J, YANG X Z, et al. Glutathione-dependent induction of local and systemic defense against oxidative stress by exogenous melatonin in cucumber (Cucumis sativus L. ) [J]. Journal of Pineal Research, 2016, 60(2): 206−216. doi: 10.1111/jpi.12304 [13] FUELL C, ELLIOTT K A, HANFREY C C, et al. Polyamine biosynthetic diversity in plants and algae [J]. Plant Physiology and Biochemistry, 2010, 48(7): 513−520. doi: 10.1016/j.plaphy.2010.02.008 [14] 王学奎, 黄见良. 植物生理生化实验原理与技术[M]. 3版. 北京: 高等教育出版社, 2015: 280-281. [15] ISHITANI M, XIONG L, LEE H, et al. HOS1, a genetic locus involved in cold-responsive gene expression in Arabidopsis [J]. The Plant Cell, 1998, 10(7): 1151−1161. doi: 10.1105/tpc.10.7.1151 [16] GIANNOPOLITIS C N, RIES S K. Superoxide dismutases [J]. Plant Physiology, 1977, 59(2): 309−314. doi: 10.1104/pp.59.2.309 [17] NICKEL K S, CUNNINGHAM B A. Improved peroxidase assay method using leuco 2, 3', 6-trichloroindophenol and application to comparative measurements of peroxidatic catalysis [J]. Analytical Biochemistry, 1969, 27(2): 292−299. doi: 10.1016/0003-2697(69)90035-9 [18] DHINDSA R S, PLUMB-DHINDSA P L, REID D M. Leaf senescence and lipid peroxidation: Effects of some phytohormones, and scavengers of free radicals and singlet oxygen [J]. Physiologia Plantarum, 1982, 56(4): 453−457. doi: 10.1111/j.1399-3054.1982.tb04539.x [19] PINHERO R G, RAO M V, et al. Changes in activities of antioxidant enzymes and their relationship to genetic and paclobutrazol-induced chilling tolerance of maize seedlings [J]. Plant Physiology, 1997, 114(2): 695−704. doi: 10.1104/pp.114.2.695 [20] DUAN J J, LI J, GUO S R, et al. Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance [J]. Journal of Plant Physiology, 2008, 165(15): 1620−1635. doi: 10.1016/j.jplph.2007.11.006 [21] ZHAO F G, SUN C, LIU Y L, et al. Relationship between polyamine metabolism in roots and salt tolerance of barley seedlings [J]. Acta Botanica Sinica, 2003, 45(3): 295−300. [22] SU G X, AN Z F, ZHANG W H, et al. Light promotes the synthesis of lignin through the production of H2O2 mediated by diamine oxidases in soybean hypocotyls [J]. Journal of Plant Physiology, 2005, 162(12): 1297−1303. doi: 10.1016/j.jplph.2005.04.033 [23] COHEN S A, BIDLINGMEYER B A, TARVIN T L. PITC derivatives in amino acid analysis [J]. Nature, 1986, 320(6064): 769−770. doi: 10.1038/320769a0 [24] STEPONKUS P L. Role of the plasma membrane in freezing injury and cold acclimation [J]. Annual Review of Plant Physiology, 1984, 35: 543−584. doi: 10.1146/annurev.pp.35.060184.002551 [25] MIURA K, SHIBA H, OHTA M, et al. SlICE1 encoding a MYC-type transcription factor controls cold tolerance in tomato, Solanum lycopersicum [J]. Plant Biotechnology, 2012, 29(3): 253−260. doi: 10.5511/plantbiotechnology.12.0303a [26] DIONISIO-SESE M L, TOBITA S. Antioxidant responses of rice seedlings to salinity stress [J]. Plant Science, 1998, 135(1): 1−9. doi: 10.1016/S0168-9452(98)00025-9 [27] 刁倩楠, 范红伟, 张文献, 等. 外源物质对低温下甜瓜种子萌发和幼苗生理特性的影响 [J]. 分子植物育种, 2020, 18(21):7209−7216. doi: 10.13271/j.mpb.018.007209DIAO Q N, FAN H W, ZHANG W X, et al. Exogenous substances on seed germination, physiological characteristics of melon under chilling stress [J]. Molecular Plant Breeding, 2020, 18(21): 7209−7216.(in Chinese) doi: 10.13271/j.mpb.018.007209 [28] WANG L Y, LIU J L, WANG W X, et al. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress [J]. Photosynthetica, 2016, 54(1): 19−27. doi: 10.1007/s11099-015-0140-3 [29] 巩彪, 史庆华. 园艺作物褪黑素的研究进展 [J]. 中国农业科学, 2017, 50(12):2326−2337. doi: 10.3864/j.issn.0578-1752.2017.12.013GONG B, SHI Q H. Review of melatonin in horticultural crops [J]. Scientia Agricultura Sinica, 2017, 50(12): 2326−2337.(in Chinese) doi: 10.3864/j.issn.0578-1752.2017.12.013 [30] JAHAN M S, SHU S, WANG Y, et al. Melatonin alleviates heat-induced damage of tomato seedlings by balancing redox homeostasis and modulating polyamine and nitric oxide biosynthesis [J]. BMC Plant Biology, 2019, 19(1): 414. doi: 10.1186/s12870-019-1992-7 [31] ZHANG Q, LIU X F, ZHANG Z F, et al. Melatonin improved waterlogging tolerance in alfalfa (Medicago sativa) by reprogramming polyamine and ethylene metabolism [J]. Frontiers in Plant Science, 2019, 10: 44. doi: 10.3389/fpls.2019.00044 [32] BRETON G, DANYLUK J, CHARRON J B F, et al. Expression profiling and bioinformatic analyses of a novel stress-regulated multispanning transmembrane protein family from cereals and Arabidopsis [J]. Plant Physiology, 2003, 132(1): 64−74. doi: 10.1104/pp.102.015255 [33] 范华, 冯双庆, 赵玉梅. 黄瓜、番茄冷害以及黄瓜温度预处理与多胺的相关性 [J]. 中国农业大学学报, 1996, 1(1):108−112.FAN H, FENG S Q, ZHAO Y M. The correlation of polyamines with chilling injury of cucumber and tomato and the treatments for alleviating chilling injury [J]. Journal of China Agricultural University, 1996, 1(1): 108−112.(in Chinese) [34] SONG Y J, DIAO Q N, QI H Y. Putrescine enhances chilling tolerance of tomato (Lycopersicon esculentum Mill.) through modulating antioxidant systems [J]. Acta Physiologiae Plantarum, 2014, 36(11): 3013−3027. doi: 10.1007/s11738-014-1672-z [35] LIU J H, KITASHIBA H, WANG J, et al. Polyamines and their ability to provide environmental stress tolerance to plants [J]. Plant Biotechnology, 2007, 24(1): 117−126. doi: 10.5511/plantbiotechnology.24.117 [36] WANG J, SUN P P, CHEN C L, et al. An arginine decarboxylase gene PtADC from Poncirus trifoliata confers abiotic stress tolerance and promotes primary root growth in Arabidopsis [J]. Journal of Experimental Botany, 2011, 62(8): 2899−2914. doi: 10.1093/jxb/erq463 [37] 计淑霞, 戴绍军, 刘炜. 植物应答低温胁迫机制的研究进展 [J]. 生命科学, 2010, 22(10):1013−1019. doi: 10.13376/j.cbls/2010.10.006JI S X, DAI S J, LIU W. The advances of plants in response and adaption to low temperature stress [J]. Chinese Bulletin of Life Sciences, 2010, 22(10): 1013−1019.(in Chinese) doi: 10.13376/j.cbls/2010.10.006 [38] THOMASHOW M F. Role of cold-responsive genes in plant freezing tolerance [J]. Plant Physiology, 1998, 118(1): 1−8. doi: 10.1104/pp.118.1.1 [39] SHI Y T, DING Y L, YANG S H. Molecular regulation of CBF signaling in cold acclimation [J]. Trends in Plant Science, 2018, 23(7): 623−637. doi: 10.1016/j.tplants.2018.04.002 [40] WANG D Z, JIN Y N, DING X H, et al. Gene regulation and signal transduction in the ICE-CBF-COR signaling pathway during cold stress in plants [J]. Biochemistry Biokhimiia, 2017, 82(10): 1103−1117. doi: 10.1134/S0006297917100030 [41] MA X C, CHEN C, YANG M M, et al. Cold-regulated protein (SlCOR413IM1) confers chilling stress tolerance in tomato plants [J]. Plant Physiology and Biochemistry, 2018, 124: 29−39. doi: 10.1016/j.plaphy.2018.01.003 [42] WANG F, CHEN X X, DONG S J, et al. Crosstalk of PIF4 and DELLA modulates CBF transcript and hormone homeostasis in cold response in tomato [J]. Plant Biotechnology Journal, 2020, 18(4): 1041−1055. doi: 10.1111/pbi.13272 [43] KOU S, CHEN L, TU W, et al. The arginine decarboxylase gene ADC1, associated to the putrescine pathway, plays an important role in potato cold-acclimated freezing tolerance as revealed by transcriptome and metabolome analyses [J]. The Plant Journal, 2018, 96(6): 1283−1298. doi: 10.1111/tpj.14126 [44] KIM Y S, LEE M, LEE J H, et al. The unified ICE-CBF pathway provides a transcriptional feedback control of freezing tolerance during cold acclimation in Arabidopsis [J]. Plant Molecular Biology, 2015, 89(1/2): 187−201. [45] PARK S, LEE C M, DOHERTY C J, et al. Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network [J]. The Plant Journal, 2015, 82(2): 193−207. doi: 10.1111/tpj.12796 [46] GILMOUR S J, FOWLER S G, THOMASHOW M F. Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities [J]. Plant Molecular Biology, 2004, 54(5): 767−781. doi: 10.1023/B:PLAN.0000040902.06881.d4 [47] LI H, DING Y L, SHI Y T, et al. MPK3- and MPK6-mediated ICE1 phosphorylation negatively regulates ICE1 stability and freezing tolerance in Arabidopsis [J]. Developmental Cell, 2017, 43(5): 630−642.e4. doi: 10.1016/j.devcel.2017.09.025 [48] LIU Y K, ZHOU J. MAPping kinase regulation of ICE1 in freezing tolerance [J]. Trends in Plant Science, 2018, 23(2): 91−93. doi: 10.1016/j.tplants.2017.12.002 [49] RAMIREZ V E, POPPENBERGER B. MAP kinase signaling turns to ICE [J]. Developmental Cell, 2017, 43(5): 545−546. doi: 10.1016/j.devcel.2017.10.032 [50] WANG L, ZHAO R R, ZHENG Y Y, et al. SlMAPK1/2/3 and antioxidant enzymes are associated with H2O 2-induced chilling tolerance in tomato plants [J]. Journal of Agricultural and Food Chemistry, 2017, 65(32): 6812−6820. doi: 10.1021/acs.jafc.7b01685 [51] TAKAHASHI Y, BERBERICH T, MIYAZAKI A, et al. Spermine signalling in tobacco: Activation of mitogen-activated protein kinases by spermine is mediated through mitochondrial dysfunction [J]. The Plant Journal, 2003, 36(6): 820−829. doi: 10.1046/j.1365-313X.2003.01923.x [52] DAVLETOVA S, SCHLAUCH K, COUTU J, et al. The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis [J]. Plant Physiology, 2005, 139(2): 847−856. doi: 10.1104/pp.105.068254 [53] LI C, WANG P, WEI Z W, et al. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis [J]. Journal of Pineal Research, 2012, 53(3): 298−306. doi: 10.1111/j.1600-079X.2012.00999.x [54] ZHAO Y, TAN D X, LEI Q, et al. Melatonin and its potential biological functions in the fruits of sweet cherry [J]. Journal of Pineal Research, 2013, 55(1): 79−88. doi: 10.1111/jpi.12044 -







 下载:
下载: