Identification and Characterization of Heat Shock Protein Hsp70 in Setosphaeria turcica
-
摘要:
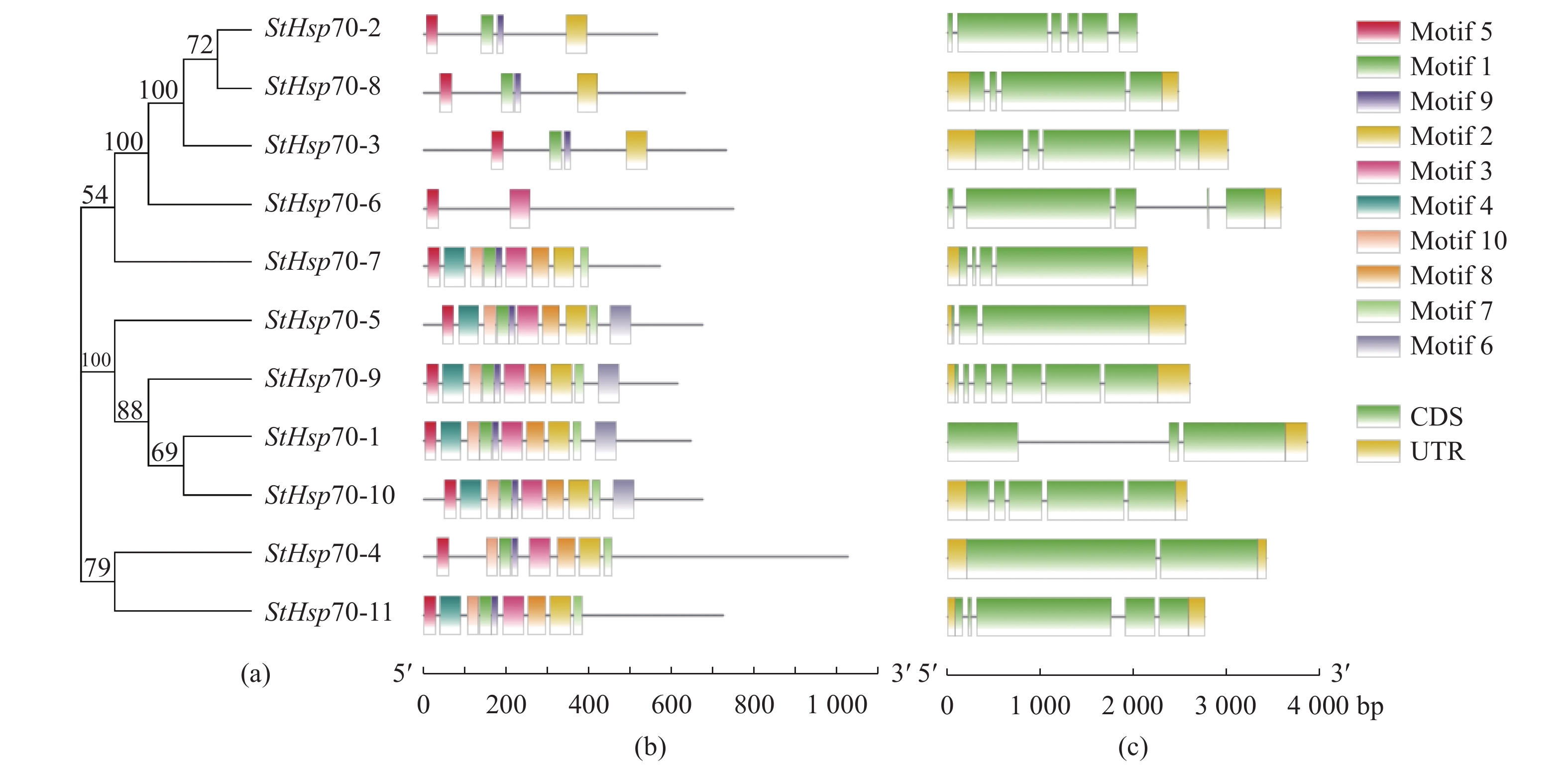
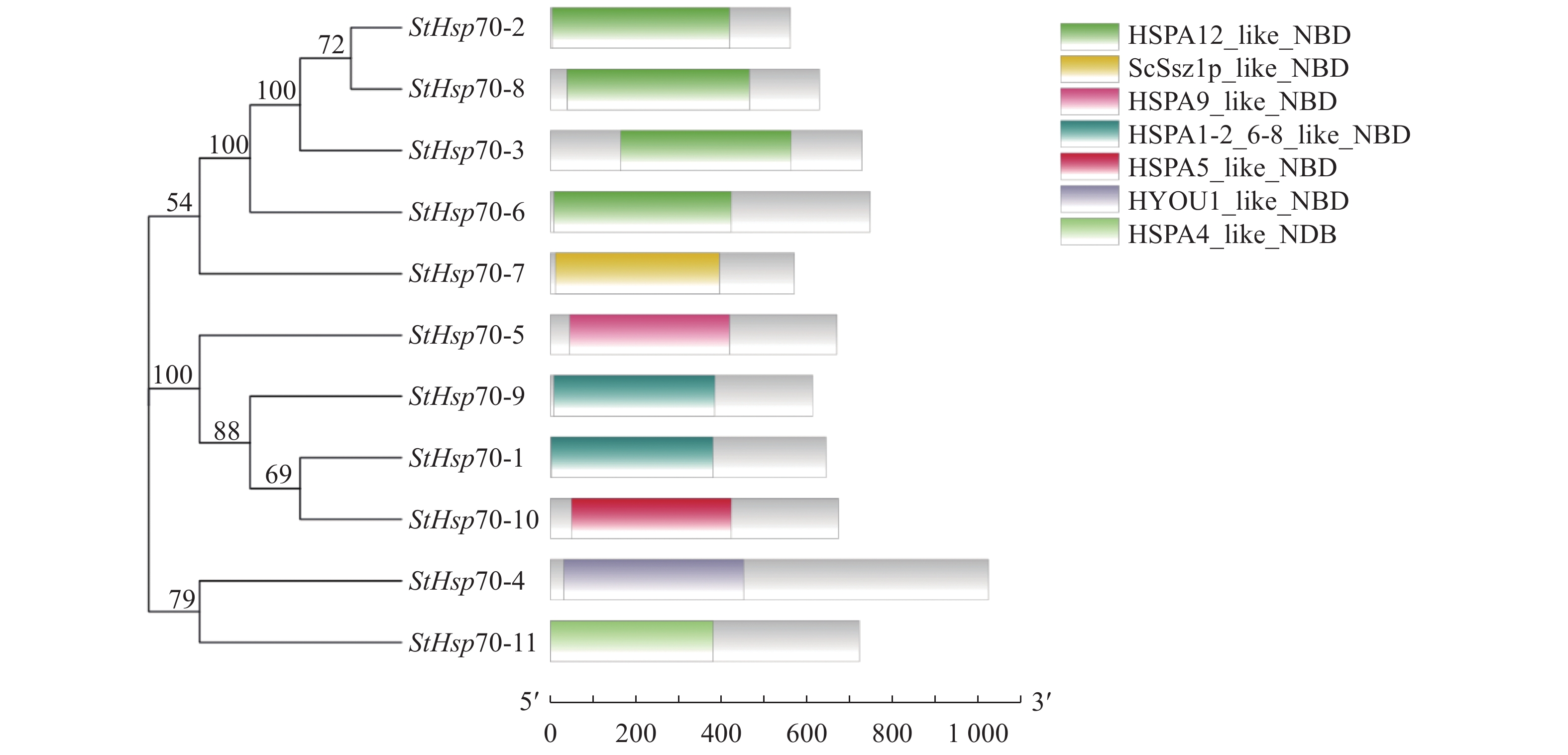
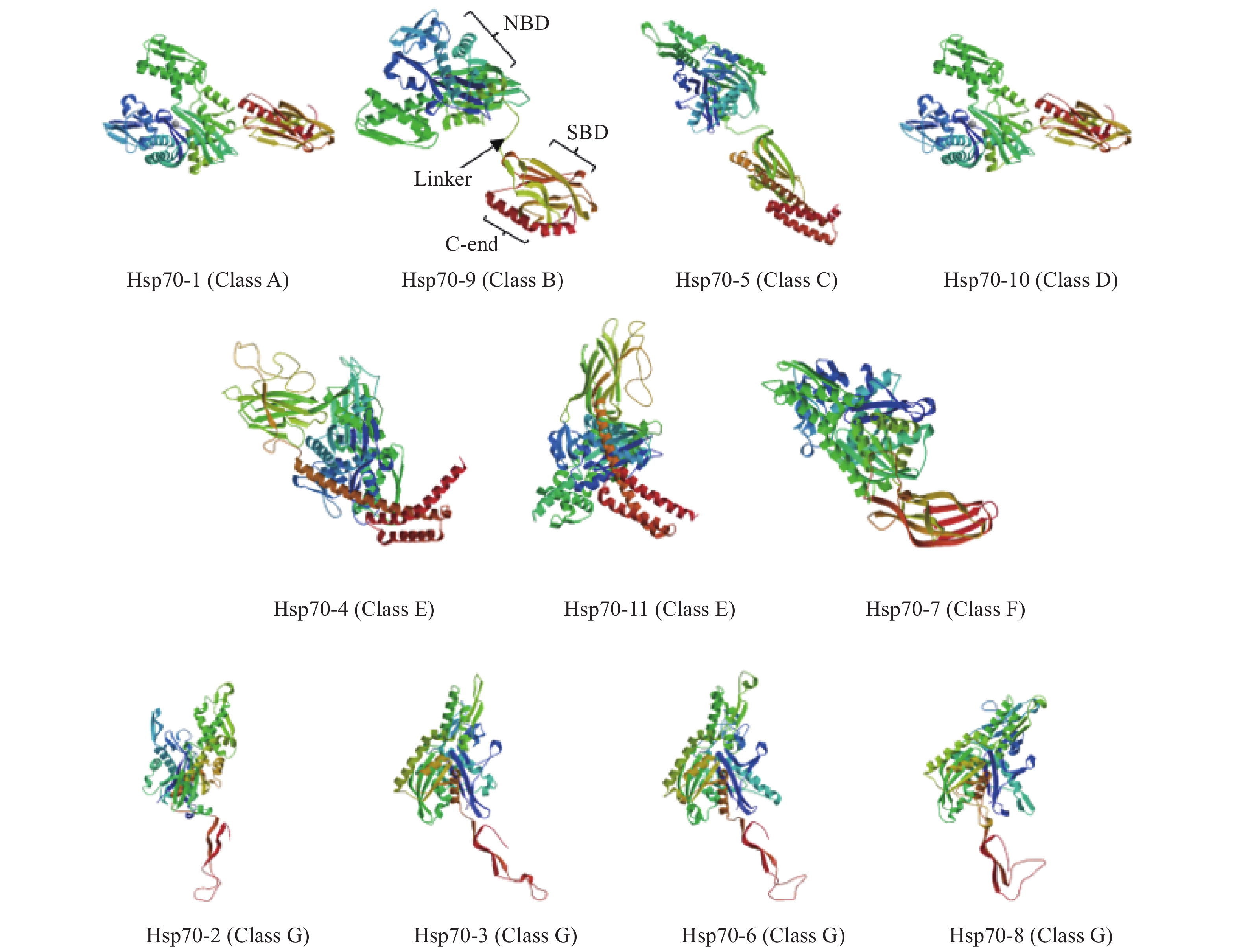
目的 对玉米大斑病菌Hsp70(Setosphaeria turcica Hsp70,StHsp70)基因家族进行结构鉴定和功能分析,为进一步阐明StHsp70在玉米大斑病菌生长发育和致病过程中的作用奠定基础。 方法 利用玉米大斑病菌基因组数据库获得StHsp70基因家族成员,利用生物信息学方法进行理化性质、亚细胞定位、系统进化、保守基序和结构域分析,构建三级结构模型,并预测启动子等顺式作用元件。 结果 StHsp70基因家族包括11个成员,分别为StHsp70-1~StHsp70-11,多数定位于细胞质,其次为内质网、线粒体和细胞核。系统进化分析结果表明,StHsp70家族成员可分为7类,其中Class A~F分别与酿酒酵母(Saccharomyces cerevisiae)热激蛋白SSA、SSB、SSC、KAR2、SSE、SSZ高度同源,而Class G在酵母中未发现其同源蛋白。结构预测分析发现,StHsp70家族成员都含有保守基序Motif 5,而Motif 6只存在于Class A~D中,Class G仅含有2~4种基序,与其他StHsp70家族成员存在显著差异。N端的NBD结构域类型在玉米大斑病菌和酵母不同Hsp70类型间存在显著差异,可能与亚细胞定位有关。不同类型StHsp70的C末端结构和延伸性变化较大,特别是Class G的StHsp70与其他类型具有显著区别,可能与底物的多样性有关。 结论 玉米大斑病菌Hsp70家族11个成员可以分为7类,其中Class G的4个成员在理化性质和结构特征上都与其他成员具有显著的区别,表明StHsp70属于多功能分子伴侣家族。 Abstract:Objective Identification and characteristics of Hsp70 family in Setosphaeria turcica were studied to facilitate elucidating their roles in the growth, development, and pathogenicity of the microbe. Methods Members of StHsp70 family were identified from the S. turcica genome database. Physicochemical properties, subcellular localization, phylogenetic evolution, conserved motifs, and domains of the genes analyzed by bioinformatics methods. Results Eleven members, StHsp70-1 to StHsp70-11, were identified from the database. Most of them were predicated to locate in the cytoplasmic as well as in endoplasmic reticulum, mitochondrial, and nucleus in lesser amounts. The phylogenetic analysis divided the members into 7 categories including Classes A−F that showed a high homology with the heat shock proteins SSA, SSB, SSC, KAR2, SSE, and SSZ of Saccharomyces cerevisiae, respectively, and Class G that had none with what were found in yeasts. All StHsp70s contained conserved motif 5, but motif 6 existed in Class A−D only. Class G had only motifs 2−4 making the class significantly different from the others. The variations in subcellular localization might be the reason of the significant N-terminal differences in the NBD domains of the Hsp70 classes of S. turcica from those of yeast. Whereas the considerably varied C-terminal structure and extensibility among the classes, especially on the StHsp70s of Class G, might contribute to the diversity of substrates. Conclusion The 11 members of Hsp70 family of S. turcica could be divided into 7 classes with 4 members in Class G being significantly different from the others in physicochemical properties and structure. It indicated that the genes were of multifunctional molecular chaperones. -
Key words:
- Setosphaeria turcica /
- Hsp70 /
- structure analysis /
- function prediction
-
表 1 玉米大斑病菌Hsp70基因家族信息
Table 1. Information on Hsp70 gene family in S. turcica
基因
Gene染色体定位
Chromosome location蛋白质ID
Protein ID氨基酸数量
The amount of amino acid分子量
Molecular weight等电点
pI亚细胞定位
Subcellular
localizationHsp70-1 scaffold_1 133966 645 70064 5.10 细胞质Cytoplasmic Hsp70-2 scaffold_1 86462 562 62877 8.06 细胞质Cytoplasmic
线粒体MitochondrialHsp70-3 scaffold_3 163910 729 80129 8.30 细胞核Nuclear Hsp70-4 scaffold_3 163930 1025 111897 5.94 内质网Endoplasmic reticulum
细胞质CytoplasmicHsp70-5 scaffold_4 164298 671 72827 5.62 线粒体Mitochondrial Hsp70-6 scaffold_4 1352336 748 84696 6.14 细胞质Cytoplasmic Hsp70-7 scaffold_8 122795 571 61047 5.05 细胞质Cytoplasmic Hsp70-8 scaffold_10 166606 629 69891 6.49 细胞质Cytoplasmic Hsp70-9 scaffold_14 168163 614 66956 5.35 细胞质Cytoplasmic Hsp70-10 scaffold_16 168574 674 73861 5.09 内质网Endoplasmic reticulum Hsp70-11 scaffold_18 168841 722 80441 5.23 细胞质Cytoplasmic -
[1] HENDRICK J P, HARTL F U. Molecular chaperone functions of heat-shock proteins [J]. Heredity, 1993, 62: 349−384. [2] FARHAN Y ALMALKI A, ARABDIN M, KHAN A. The role of heat shock proteins in cellular homeostasis and cell survival [J]. Cureus, 2021, 13(9): e18316. [3] SHAN Q, MA F, WEI J, et al. Physiological functions of heat shock proteins [J]. Current Protein & Peptide Science, 2020, 21(8): 751−760. [4] CARPANE P D, PEPER A M, KOHN F. Management of northern corn leaf blight using Nativo (Trifloxistrobin + Tebuconazole) fungicide applications [J]. Crop Protection, 2020, 127(C): 104982. [5] 李茂盛. 玉米大斑病的发生规律及防治 [J]. 吉林农业, 2019(5):69. doi: 10.14025/j.cnki.jlny.2019.05.029LI M S. Occurrence regularity and control of corn leaf blight [J]. Agriculture of Jilin, 2019(5): 69.(in Chinese) doi: 10.14025/j.cnki.jlny.2019.05.029 [6] 王彩霞. 玉米大斑病的发病原因及防治策略 [J]. 南方农业, 2021, 15(3):48−49. doi: 10.19415/j.cnki.1673-890x.2021.03.022WANG C X. The cause and control strategy of Cercospora Maydis [J]. South China Agriculture, 2021, 15(3): 48−49.(in Chinese) doi: 10.19415/j.cnki.1673-890x.2021.03.022 [7] MORIMOTO R I, TISSIERES A, GEORGOPOULOS C. The stress response, function of the proteins and perspectives [J]. Cold Spring Harbor monograph archive, 1990, 19: 1−36. [8] FINKA A, MATTOO R U H, GOLOUBINOFF P. Experimental milestones in the discovery of molecular chaperones as polypeptide unfolding enzymes [J]. Annual Review of Biochemistry, 2016, 85(1): 715−742. doi: 10.1146/annurev-biochem-060815-014124 [9] BERKA M, KOPECKÁ R, BERKOVÁ V, et al. Regulation of heat shock proteins 70 and their role in plant immunity [J]. Journal of Experimental Botany, 2022, 73(7): 1894−1909. doi: 10.1093/jxb/erab549 [10] CHASTON J J, SMITS C, ARAGÃO D, et al. Structural and functional insights into the evolution and stress adaptation of type II chaperonins [J]. Structure, 2016, 24(3): 364−374. doi: 10.1016/j.str.2015.12.016 [11] SEO K, CHOI E, LEE D, et al. Heat shock factor 1 mediates the longevity conferred by inhibition of TOR and insulin/IGF-1 signaling pathways in C. elegans [J]. Aging Cell, 2013, 12(6): 1073−1081. doi: 10.1111/acel.12140 [12] WEGRZYN R D, DEUERLING E. Molecular guardians for newborn proteins: Ribosome-associated chaperones and their role in protein folding [J]. Cellular and Molecular Life Sciences, 2005, 62(23): 2727−2738. doi: 10.1007/s00018-005-5292-z [13] WERNER-WASHBURNE M, STONE D E, CRAIG E A. Complex interactions among members of an essential subfamily of hsp70 genes in Saccharomyces cerevisiae [J]. Molecular and Cellular Biology, 1987, 7(7): 2568−2577. [14] XU X P, SARBENG E B, VORVIS C, et al. Unique peptide substrate binding properties of 110-kDa heat-shock protein (Hsp110) determine its distinct chaperone activity [J]. The Journal of Biological Chemistry, 2012, 287(8): 5661−5672. doi: 10.1074/jbc.M111.275057 [15] DRAGOVIC Z, BROADLEY S A, SHOMURA Y, et al. Molecular chaperones of the Hsp110 family act as nucleotide exchange factors of Hsp70s [J]. The EMBO Journal, 2006, 25(11): 2519−2528. doi: 10.1038/sj.emboj.7601138 [16] GAUTSCHI M, LILIE H, FÜNFSCHILLING U, et al. RAC, a stable ribosome-associated complex in yeast formed by the DnaK-DnaJ homologs Ssz1p and zuotin [J]. Proceedings of the National Academy of Sciences of the United States of America, 2001, 98(7): 3762−3767. doi: 10.1073/pnas.071057198 [17] HALLSTROM T C, MOYE-ROWLEY W S. Hyperactive forms of the Pdr1p transcription factor fail to respond to positive regulation by the hsp70 protein Pdr13p [J]. Molecular Microbiology, 2000, 36(2): 402−413. doi: 10.1046/j.1365-2958.2000.01858.x [18] EISENMAN H C, CRAIG E A. Activation of pleiotropic drug resistance by the J-protein and Hsp70-related proteins, Zuo1 and Ssz1 [J]. Molecular Microbiology, 2004, 53(1): 335−344. doi: 10.1111/j.1365-2958.2004.04134.x [19] MONTERO-BARRIENTOS M, HERMOSA R, NICOLÁS C, et al. Overexpression of a Trichoderma HSP70 gene increases fungal resistance to heat and other abiotic stresses [J]. Fungal Genetics and Biology, 2008, 45(11): 1506−1513. doi: 10.1016/j.fgb.2008.09.003 [20] 金承涛, 曾云中, 吴雪昌, 朱旭芬. 耐热酵母菌株HU-TY-1的耐热机理初探 [J]. 浙江大学学报(理学版), 2001, 28(6):676−681.JIN C T, ZENG Y Z, WU X C, et al. Study on heat shock protein and thermotolerant mechanism of S. cerevisiae [J]. Journal of Zhejiang University (Sciences Edition), 2001, 28(6): 676−681.(in Chinese) [21] 谢翎, 陈红梅, 汤强, 等. 实时荧光定量PCR检测球孢白僵菌热休克蛋白基因hsp70在几种胁迫条件下的表达 [J]. 菌物学报, 2009, 28(6):806−812. doi: 10.13346/j.mycosystema.2009.06.013XIE L, CHEN H M, TANG Q, et al. Expression analysis of hsp70 gene from Beauveria bassiana under several stress conditions by Realtime-PCR [J]. Mycosystema, 2009, 28(6): 806−812.(in Chinese) doi: 10.13346/j.mycosystema.2009.06.013 [22] 曹华宁, 刘博, 刘太国, 等. 小麦条锈菌hsp70基因的克隆及热胁迫下的表达特征分析 [J]. 植物保护, 2015, 41(3):19−24. doi: 10.3969/j.issn.0529-1542.2015.03.004CAO H N, LIU B, LIU T G, et al. Cloning of a heat shock protein gene hsp70 of Puccinia striiformis f. sp. tritici and its expression in response to high-temperature stress [J]. Plant Protection, 2015, 41(3): 19−24.(in Chinese) doi: 10.3969/j.issn.0529-1542.2015.03.004 [23] YI M, CHI M H, KHANG C H, et al. The ER chaperone LHS1 is involved in asexual development and rice infection by the blast fungus Magnaporthe oryzae [J]. The Plant Cell, 2009, 21(2): 681−695. doi: 10.1105/tpc.107.055988 [24] YANG J, LIU M X, LIU X Y, et al. Heat-shock proteins MoSsb1, MoSsz1, and MoZuo1 attenuate MoMkk1-mediated cell-wall integrity signaling and are important for growth and pathogenicity of Magnaporthe oryzae [J]. Molecular Plant-Microbe Interactions, 2018, 31(11): 1211−1221. doi: 10.1094/MPMI-02-18-0052-R [25] CHEN L L, GENG X J, MA Y M, et al. The ER lumenal Hsp70 protein FpLhs1 is important for conidiation and plant infection in Fusarium pseudograminearum [J]. Frontiers in Microbiology, 2019, 10: 1401. doi: 10.3389/fmicb.2019.01401 [26] LIU Z, WANG Z, HUANG M, et al. The FgSsb-FgZuo-FgSsz complex regulates multiple stress responses and mycotoxin production via folding the soluble SNARE Vam7 and β2-tubulin in Fusarium graminearum [J]. Environmental Microbiology, 2017, 19(12): 5040−5059. doi: 10.1111/1462-2920.13968 [27] STONE D E, CRAIG E A. Self-regulation of 70-kilodalton heat shock proteins in Saccharomyces cerevisiae [J]. Molecular and Cellular Biology, 1990, 10(4): 16222−1632. [28] MURAKAMI H, PAIN D, BLOBEL G. 70-kD heat shock-related protein is one of at least two distinct cytosolic factors stimulating protein import into mitochondria [J]. Revista Espanola De Enfermedades Digestivas, 1988, 107(6 pt 1): 2051−2057. [29] XU C L, WANG S, THIBAULT G, et al. Futile protein folding cycles in the ER are terminated by the unfolded protein O-mannosylation pathway [J]. Science, 2013, 340(6135): 978−981. doi: 10.1126/science.1234055 [30] NISHIKAWA S I, FEWELL S W, KATO Y, et al. Molecular chaperones in the yeast endoplasmic reticulum maintain the solubility of proteins for retrotranslocation and degradation [J]. Scientific Reports, 2001, 153(5): 1061−1070. [31] LYMAN S K, SCHEKMAN R. Binding of secretory precursor polypeptides to a translocon sub complex is regulated by BiP [J]. Cell, 1997, 88(1): 85−96. doi: 10.1016/S0092-8674(00)81861-9 [32] HUANG P, GAUTSCHI M, WALTER W, et al. The Hsp70 Ssz1 modulates the function of the ribosome-associated J-protein Zuo1 [J]. Nature Structural & Molecular Biology, 2005, 12(6): 497−504. [33] CUI Z M, WANG P, SUN L L, et al. Lipopolysaccharide-evoked HSPA12B expression by activation of MAPK cascade in microglial cells of the spinal cord [J]. Journal of the Neurological Sciences, 2010, 294(1/2): 29−37. [34] SARKAR N K, KUNDNANI P, GROVER A. Functional analysis of Hsp70 superfamily proteins of rice (Oryza sativa) [J]. Cell Stress & Chaperones, 2013, 18(4): 427−437. -







 下载:
下载: