Expressions of Defense Signal Pathway Genes in Tomato Plant Induced by Ralstonia solanacearum of Different Virulence
-
摘要:
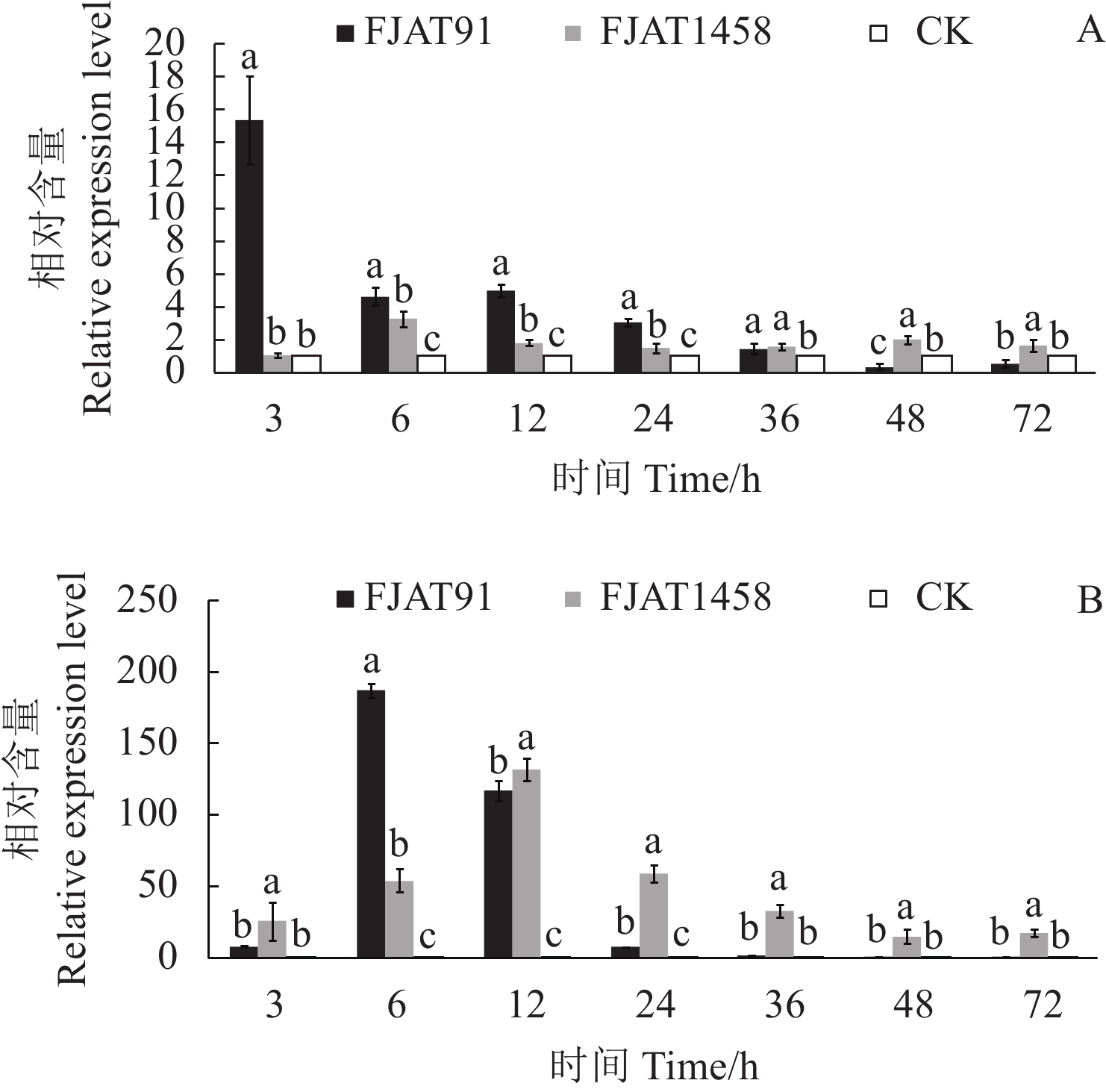
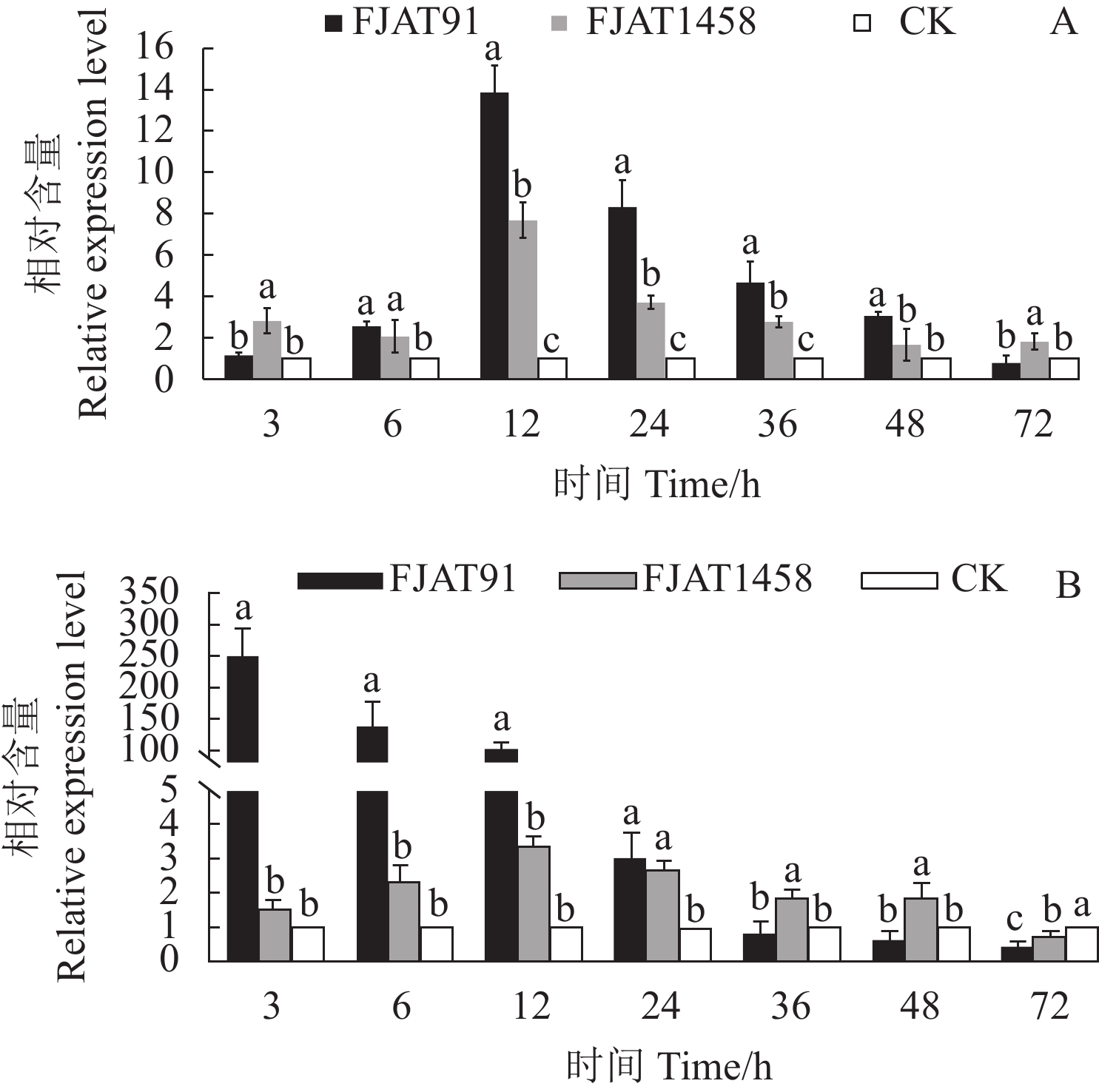
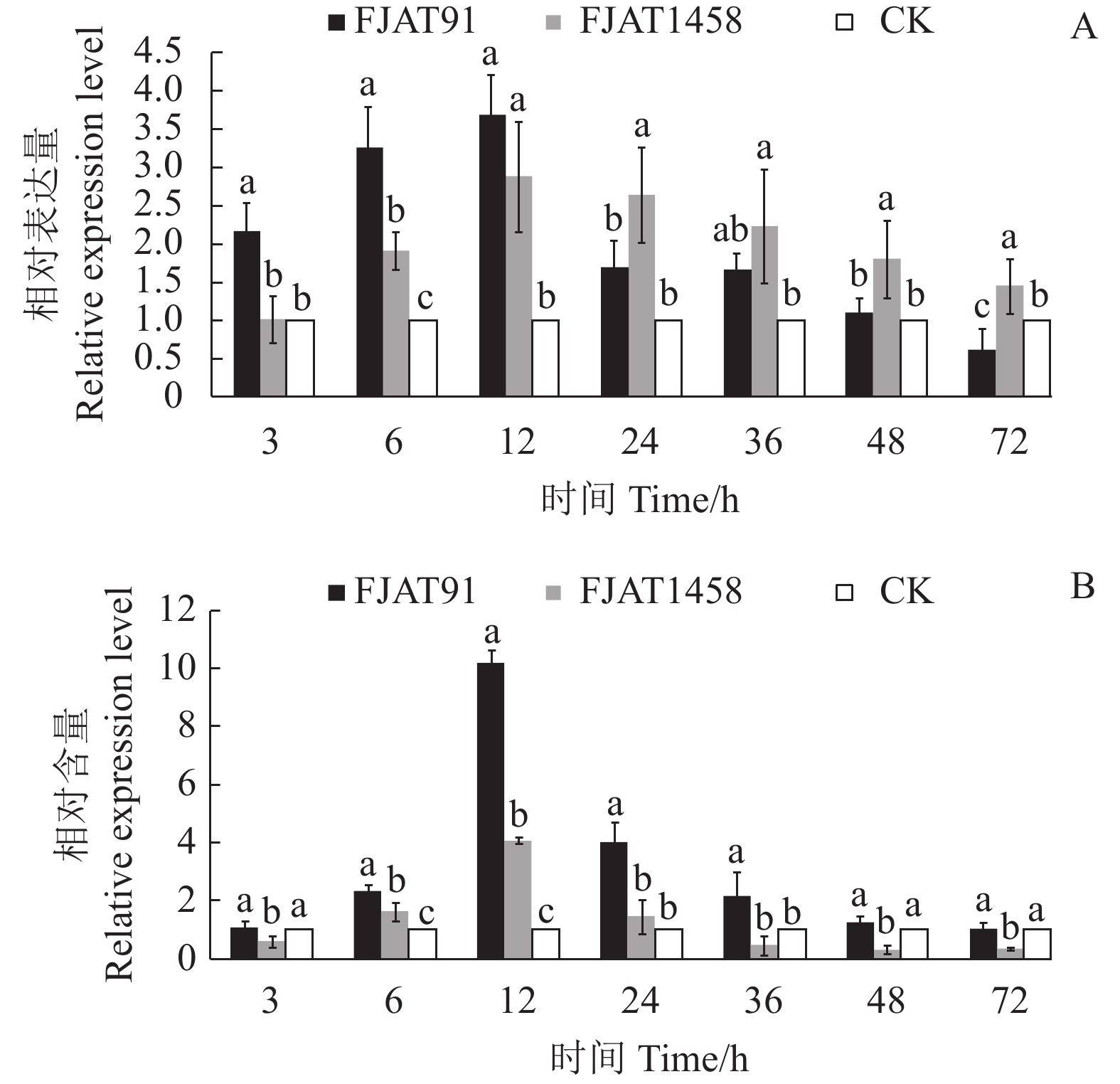
目的 探究不同致病力青枯雷尔氏菌诱导番茄防御相关基因的表达水平,为探明无致病力青枯雷尔氏菌的生防机制奠定基础。 方法 以无菌水为对照(CK),分别接种青枯雷尔氏菌强致病力菌株FJAT91和无致病力菌株FJAT1458,接种后不同时间(0 ,3,6 ,12 ,24 ,36 ,48 ,72 h)取样,利用荧光定量PCR技术,检测接种不同致病力青枯雷尔氏菌后番茄植株中水杨酸(SA)信号转导途径的gluA和PR-1a基因、茉莉酸(JA)信号转导途径的loxA和pin2基因及乙烯(ET)信号转导途径的Osm和PR-1a基因相对表达量的变化。 结果 菌株FJAT1458和FJAT91均能诱导番茄gluA、PR-1a、loxA、pin2、Osm和PR-1a基因的表达,接种后期(36 h之后),这些基因(PR-1a和Osm除外)在FJAT1458处理组的表达量均比FJAT91处理组高;FJAT1458和FJAT91诱导gluA和PR-1A基因表达量呈“先上升后下降”的趋势,接种12 h表达量最大;FJAT1458诱导番茄植株loxA和pin2基因表达量均显著高于CK,FJAT91诱导番茄植株loxA基因和pin2基因表达量在接种初期显著高于CK和FJAT1458处理组,后期迅速降低;FJAT-91诱导番茄植株Osm基因显著高于FJAT1458和对照处理(72 h除外),该菌株诱导番茄植株PR-1b基因表达量变化较为强烈,接种3 h,其诱导的PR-1b基因表达量为CK的251.33倍。 结论 不同致病力青枯雷尔氏菌诱导番茄防御相关基因表达量不同,可以进一步通过转录组法挖掘更多差异表达的基因。 Abstract:Objective Expressions of the defense-related genes in tomato plant induced by Ralstonia solanacearum of varied pathogenicity were studied to understand the biocontrol mechanisms of the avirulent FJAT1458. Method Real-time PCR was used to determine the expressions of gluA and PR-1a in the salicylic acid signal pathway, loxA and pin2 in the jasmonic acid signal pathway, and Osm and PR-1a in the ethylene signal pathway in tomato plants inoculated with the virulent strain FJAT91, the avirulent strain FJAT1458, or sterile water (CK) for 0, 3, 6, 12, 24, 36, 48, and 72 h. Result Both avirulent and virulent strains could induce expressions of gluA, PR-1a, loxA, pin2, Osm, and PR-1a. However, except for PR-1a and Osm, the gene expressions induced by FJAT1458 were significantly higher than those by FJAT91. Both FJAT1458 and FJAT91 inoculations raised the expressions of gluA and PR-1a to a peak followed by a decline in 12 h. The expressions of loxA and pin2 induced by FJAT1458 induction were significantly higher than CK, but FJAT91 did only initially. On the other hand, FJAT91 produced a significantly higher Osm expression than either FJAT1458 or CK, excluding the 72 h inoculation. And in the 3 h inoculation it rendered a PR-1b expression significantly stronger than FJAT1458 at 260.46 times higher than CK. Conclusion Both avirulent and virulent R. solanacearum could induce expressions of the defense-related genes in tomato plants in varied degrees. The transcriptomic method applied in this study could conceivably be used to unveil additional differently expressed genes associated with the pathways in tomato plants in the future. -
表 1 RT-PCR检测基因的特异引物序列
Table 1. Specific primer sequences used for RT-PCR
检测基因
Tested
gene信号通路
Signal pathways基因库
登记号
GenBank accession no.引物序列
Primer sequencegIuA SA M80604 F: TCAGCAGGGTTGCAAAATCA R: CTCTAGGTGGGTAGGTGTTGGTTAA PR-1a SA M69247 F: TCT TGT GAG GCC CAA AAT TC R: ATA GTC TGG CCT CTC GGA CA loxA JA U09026 F: TGGTAGACCACCAACACGAA R: GACCAAAACGCTCGTCTCTC pin2 JA AY129402 F: TGATGCCAAGGCTTGTACTAGAGA R: AGCGGACTTCCTTCTGAACGT PR-1b ET X14065 F: CCA AGA CTA TCT TGC GGT TC R: GAA CCT AAG CCA CGA TAC CA Osmotin-like ET M21346 F: TGTACCACGTTTGGAGGACA R: ACCAGGGCAAGTAAATGTGC -
[1] HAYWARD A C. Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum [J]. Annual Review of Phytopathology, 1991, 29: 65−87. doi: 10.1146/annurev.py.29.090191.000433 [2] PRIOR P, AILLOUD F, DALSING B L, et al. Genomic and proteomic evidence supporting the division of the plant pathogen Ralstonia solanacearum into three species [J]. BMC Genomics, 2016, 17: 90. doi: 10.1186/s12864-016-2413-z [3] SUGA Y, HORITA M, UMEKITA M, et al. Pathogenic characters of Japanese potato strains of Ralstonia solanacearum [J]. Journal of General Plant Pathology, 2013, 79(2): 110−114. doi: 10.1007/s10327-013-0429-7 [4] CELLIER G, PRIOR P. Deciphering phenotypic diversity of Ralstonia solanacearum strains pathogenic to potato [J]. Phytopathology, 2010, 100(11): 1250−1261. doi: 10.1094/PHYTO-02-10-0059 [5] BALABEL N M, EWED W E, MOSTAPHA M I, et al. Some epidemiological aspects of Ralstonia solanacearum [J]. Egypt Journal of Agriculture Research, 2005, 83(4): 1547−1563. [6] FREY P, PRIOR P, MARIE C, et al. Hrp mutants of Pseudomonas solanacearum as potential biocontrol agents of tomato bacterial wilt [J]. Applied and Environmental Microbiology, 1994, 60(9): 3175−3181. doi: 10.1128/aem.60.9.3175-3181.1994 [7] 杨宇红, 刘俊平, 杨翠荣, 等. 无致病力hrp-突变体防治茄科蔬菜青枯病 [J]. 植物保护学报, 2008, 35(5):433−437. doi: 10.3321/j.issn:0577-7518.2008.05.010YANG Y H, LIU J P, YANG C R, et al. Control of Solanacearum vegetable bacterial wilt with avirulent hrp- mutants [J]. Acta Phytophylacica Sinica, 2008, 35(5): 433−437.(in Chinese) doi: 10.3321/j.issn:0577-7518.2008.05.010 [8] 刘波, 蓝江林, 朱育菁, 等. 植物免疫系统的研究与应用 [J]. 中国农学通报, 2007, 23(S):163−172.LIU B, LAN J L, ZHU Y J, et al. Study and application of Plant immune system [J]. Chinese Agricultural Science Bulletin, 2007, 23(S): 163−172.(in Chinese) [9] FENG D X, TASSET C, HANEMIAN M, et al. Biological control of bacterial wilt in Arabidopsis thaliana involves abscissic acid signalling [J]. The New Phytologist, 2012, 194(4): 1035−1045. doi: 10.1111/j.1469-8137.2012.04113.x [10] PARROTT D L, HUANG L, FISCHER A M. Downregulation of a barley (Hordeum vulgare) leucine-rich repeat, non-arginine-aspartate receptor-like protein kinase reduces expression of numerous genes involved in plant pathogen defense [J]. Plant Physiology and Biochemistry, 2016, 100: 130−140. doi: 10.1016/j.plaphy.2016.01.005 [11] BLOCK A, SCHMELZ E, O'DONNELL P J, et al. Systemic acquired tolerance to virulent bacterial pathogens in tomato [J]. Plant Physiology, 2005, 138(3): 1481−1490. doi: 10.1104/pp.105.059246 [12] THALER J S, OWEN B, HIGGINS V J. The role of the jasmonate response in plant susceptibility to diverse pathogens with a range of lifestyles [J]. Plant Physiology, 2004, 135(1): 530−538. doi: 10.1104/pp.104.041566 [13] ISHIHARA T, MITSUHARA I, TAKAHASHI H, et al. Transcriptome analysis of quantitative resistance-specific response upon Ralstonia solanacearum infection in tomato [J]. Plos One, 2012, 7(10): e46763. doi: 10.1371/journal.pone.0046763 [14] CHEN Y N, REN X P, ZHOU X J, et al. Dynamics in the resistant and susceptible peanut (Arachis hypogaea L. ) root transcriptome on infection with the Ralstonia solanacearum [J]. BMC Genomics, 2014, 15: 1078. doi: 10.1186/1471-2164-15-1078 [15] ZULUAGA A P, SOLÉ M, LU H B, et al. Transcriptome responses to Ralstonia solanacearum infection in the roots of the wild potato Solanum commersonii [J]. BMC Genomics, 2015, 16: 246. doi: 10.1186/s12864-015-1460-1 [16] 郑雪芳, 刘波, 林乃铨, 等. 青枯雷尔氏菌无致病力突变菌株的构建及其防效评价模型分析 [J]. 植物病理学报, 2013, 43(5):518−531.ZHENG X F, LIU B, LIN N Q, et al. Construction of Ralstonia solanacearum avirulent mutants and evaluation model of their control efficacy against tomato bacterial wilt disease [J]. Acta Phytopathologica Sinica, 2013, 43(5): 518−531.(in Chinese) [17] 郑雪芳, 朱育菁, 刘波, 等. 番茄青枯病植物疫苗胶悬菌剂的制备及其对病害的防治效果 [J]. 植物保护, 2017, 43(2):208−211. doi: 10.3969/j.issn.0529-1542.2017.02.037ZHENG X F, ZHU Y J, LIU B, et al. Preparation of colloidal suspension agent used as plant vaccine against tomato bacterial wilt disease and its control efficacy [J]. Plant Protection, 2017, 43(2): 208−211.(in Chinese) doi: 10.3969/j.issn.0529-1542.2017.02.037 [18] KELMAN A. The relationship of pathogenicity in Pseudomonas solanacearum to colony appearance on a tetrazolium medium [J]. Phytopathology, 1954, 44: 693−695. [19] SCHMITTGEN T D, LIVAK K J. Analyzing real-time PCR data by the comparative C(T) method [J]. Nature Protocols, 2008, 3(6): 1101−1108. doi: 10.1038/nprot.2008.73 [20] 陈达. 拮抗菌和青枯菌无致病力突变株防控茄科作物青枯病的效应和机理研究[D]. 南京: 南京农业大学, 2014.CHEN D. The control efficacy of bacterial wilt in solanaceae crops by antagaonistic bacterium and avirulent mutants of Ralstonia solanacearum and mechanisms [D]. Nanjing: Nanjing agricultural university, 2014. (in Chinese) [21] 刘俊平. 无致病力hrp-突变体防治番茄青枯病作用研究 [D]. 北京: 中国农业科学院, 2006.LIU J P. Study on the control effect against tomato bacterial wilt with avirulent hrp- mutants [D]. Beijing: Chinese academy of agricultural sciences, 2006. (in Chinese) [22] MILLING A, BABUJEE L, ALLEN C. Ralstonia solanacearum extracellular polysaccharide is a specific elicitor of defense responses in wilt-resistant tomato plants [J]. PLoS One, 2011, 6(1): e15853. doi: 10.1371/journal.pone.0015853 [23] CHEN D, LI C, WU K, et al. A PhcA- marker-free mutant of Ralstonia Solanacearum as potential biocontrol agent of tomato bacterial wilt [J]. Biological Control, 2015, 80: 96−102. doi: 10.1016/j.biocontrol.2014.09.005 [24] CIARDI J A, TIEMAN D M, LUND S T, et al. Response to Xanthomonas campestris pv. Vesicatoria in tomato involves regulation of ethylene receptor gene expression [J]. Plant Physiology, 2000, 123(1): 81−92. doi: 10.1104/pp.123.1.81 -







 下载:
下载: