Cryopreservation of Malus Red Splendor Crabapple Shoots
-
摘要:
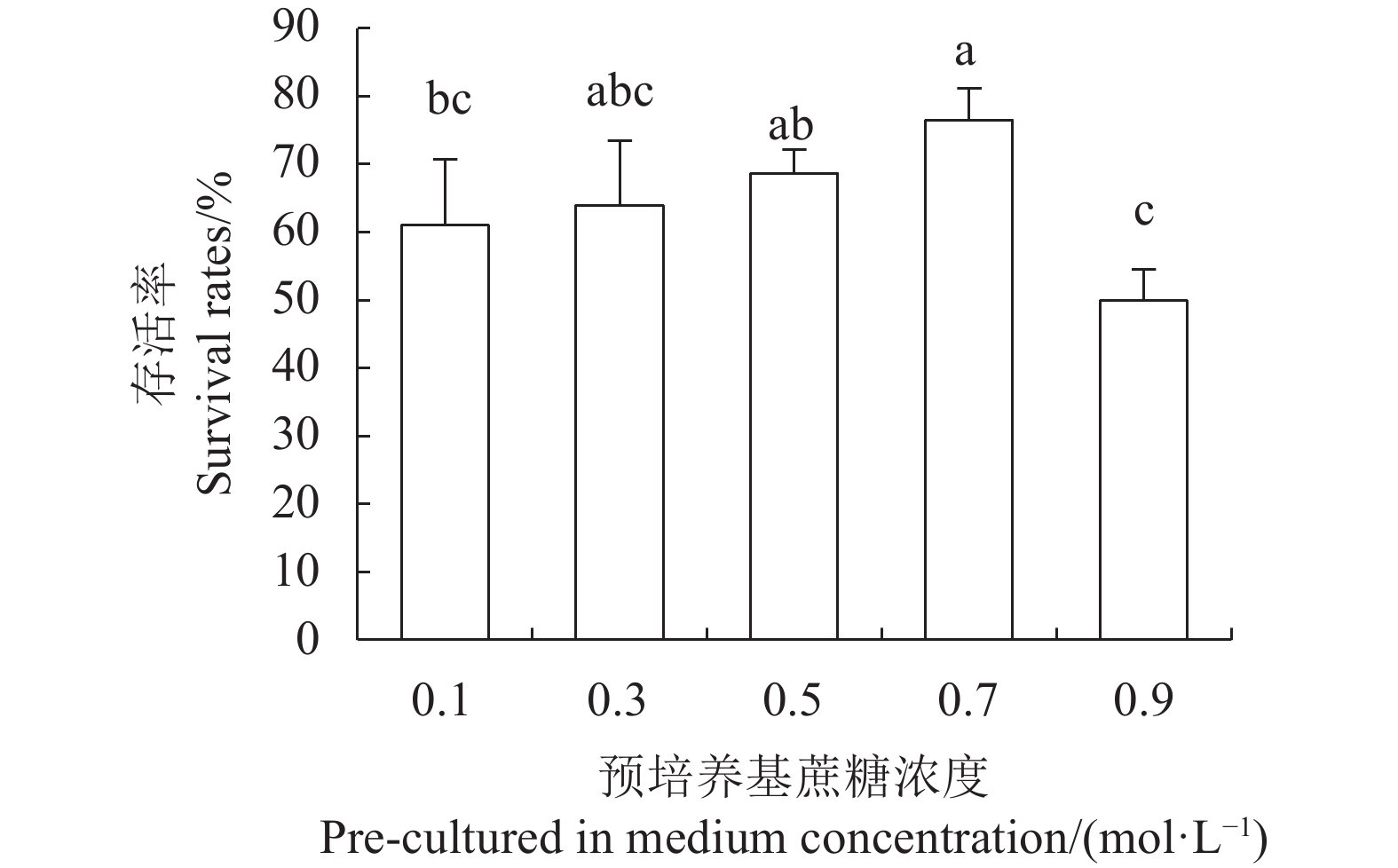
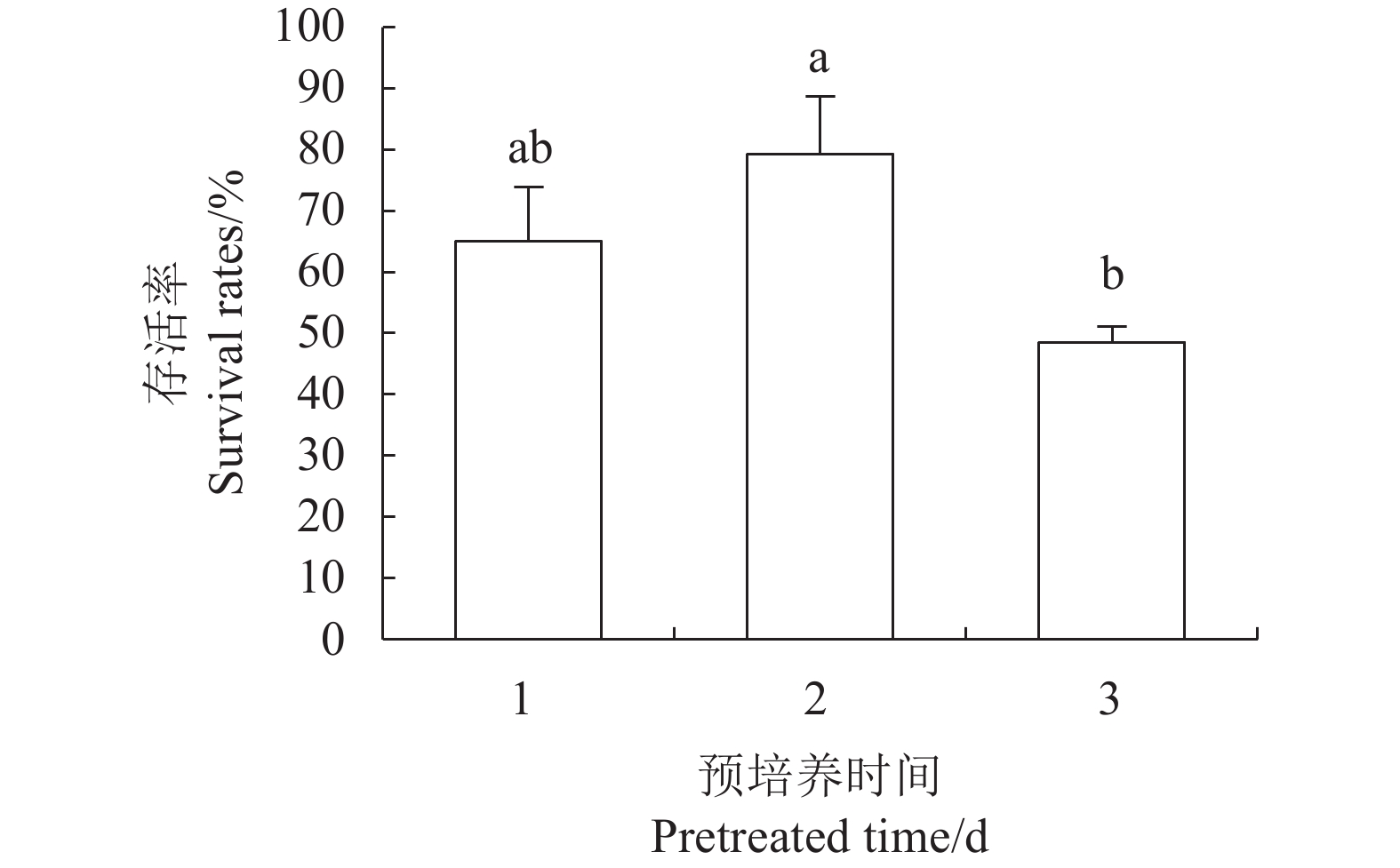
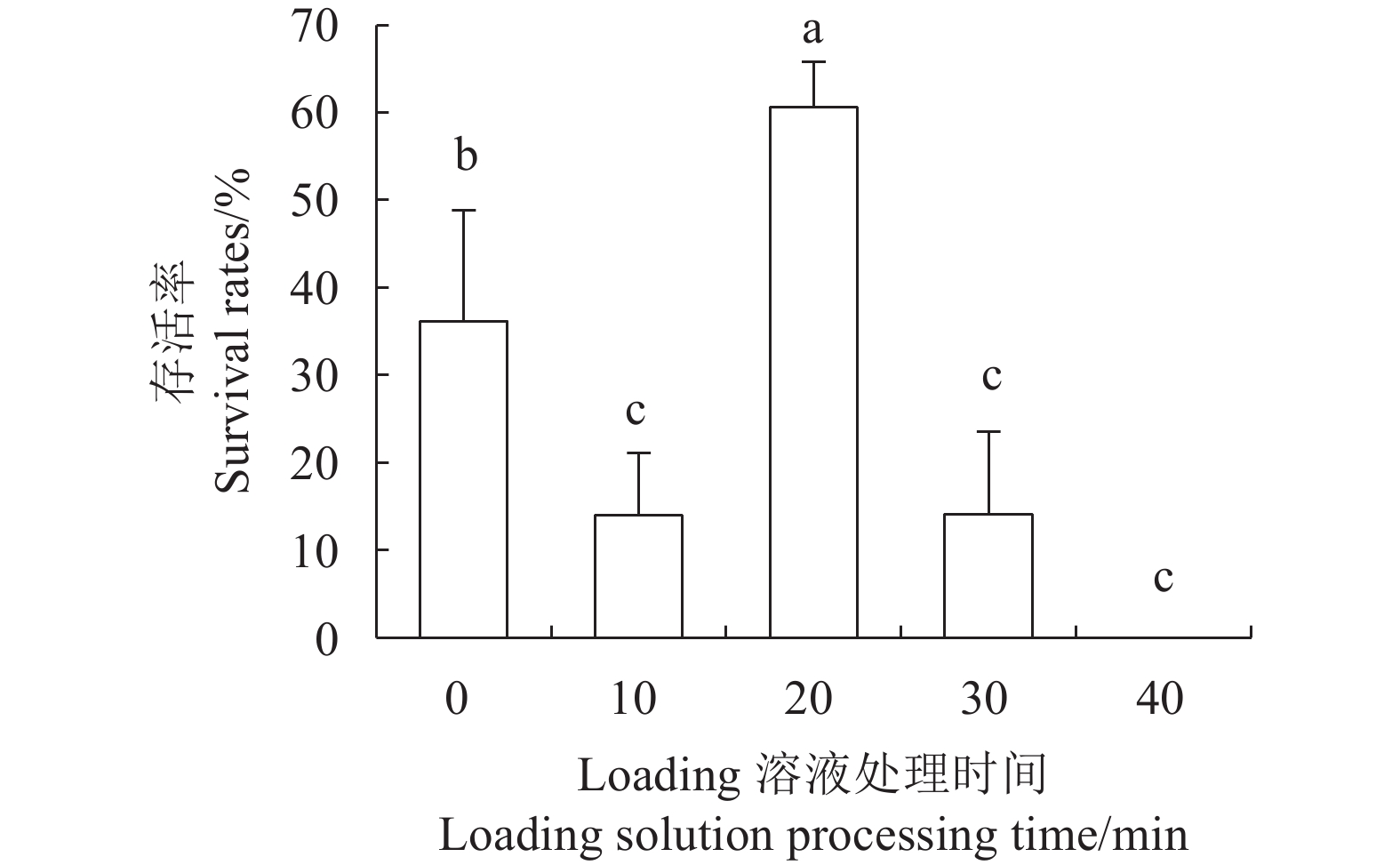
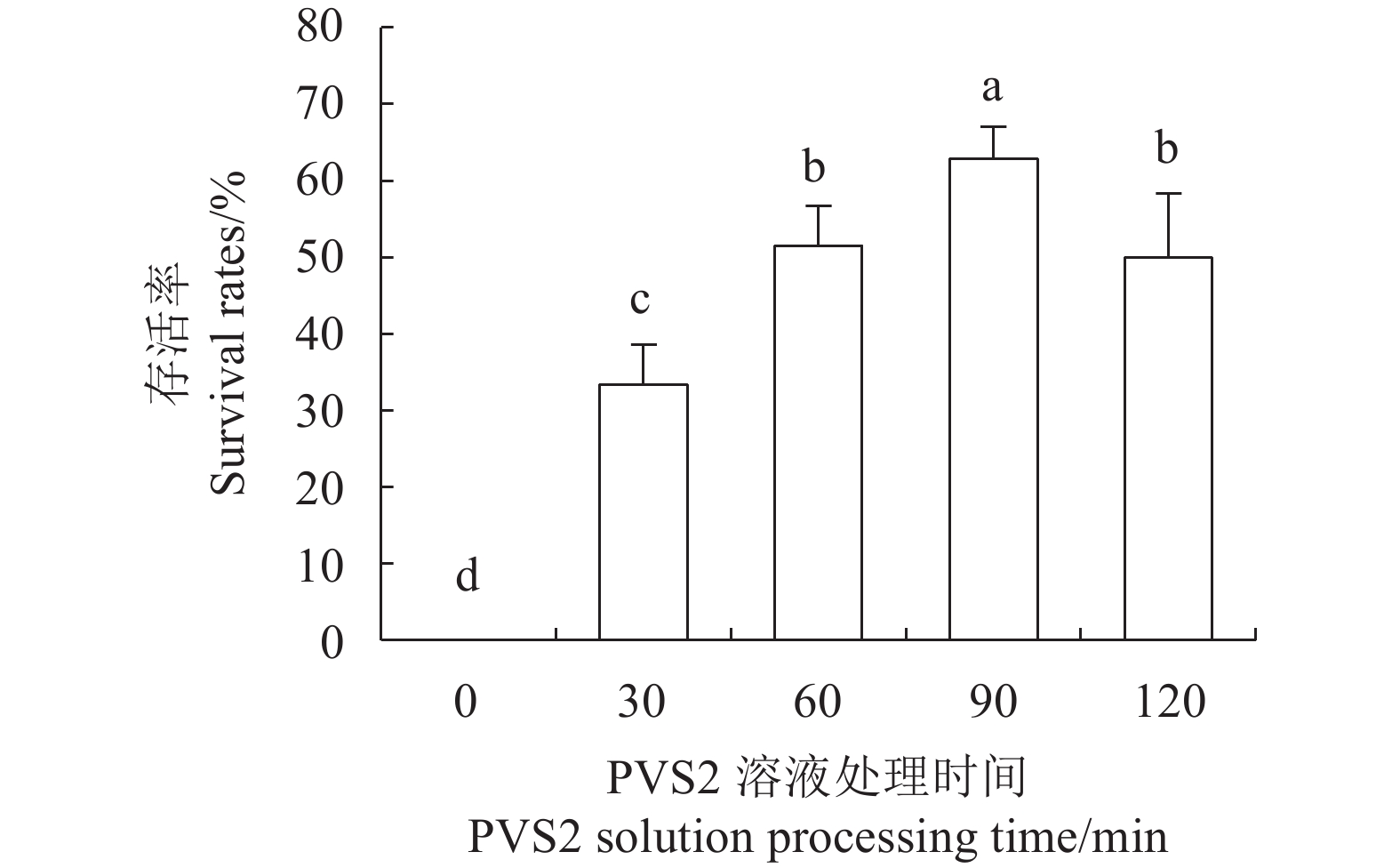
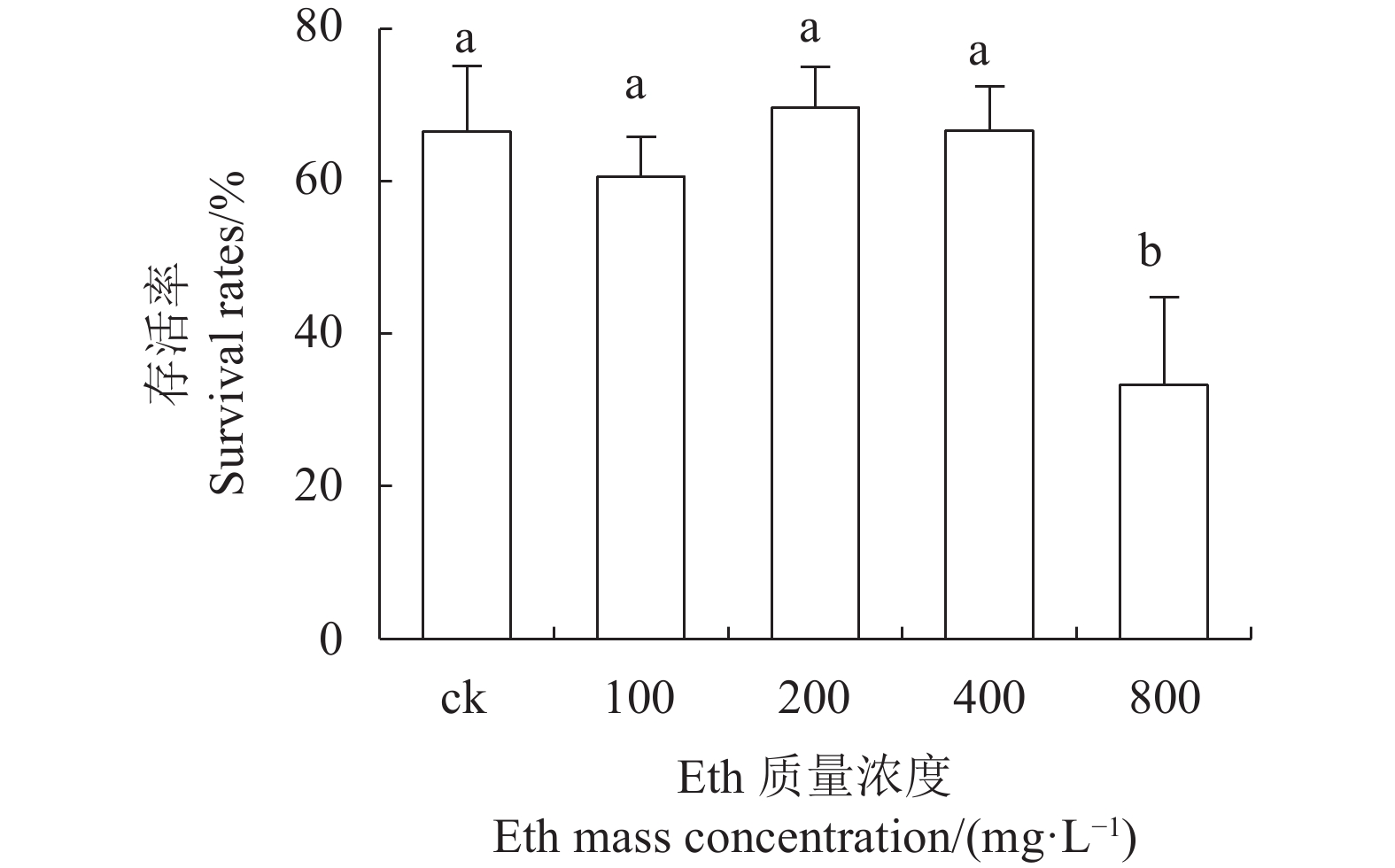
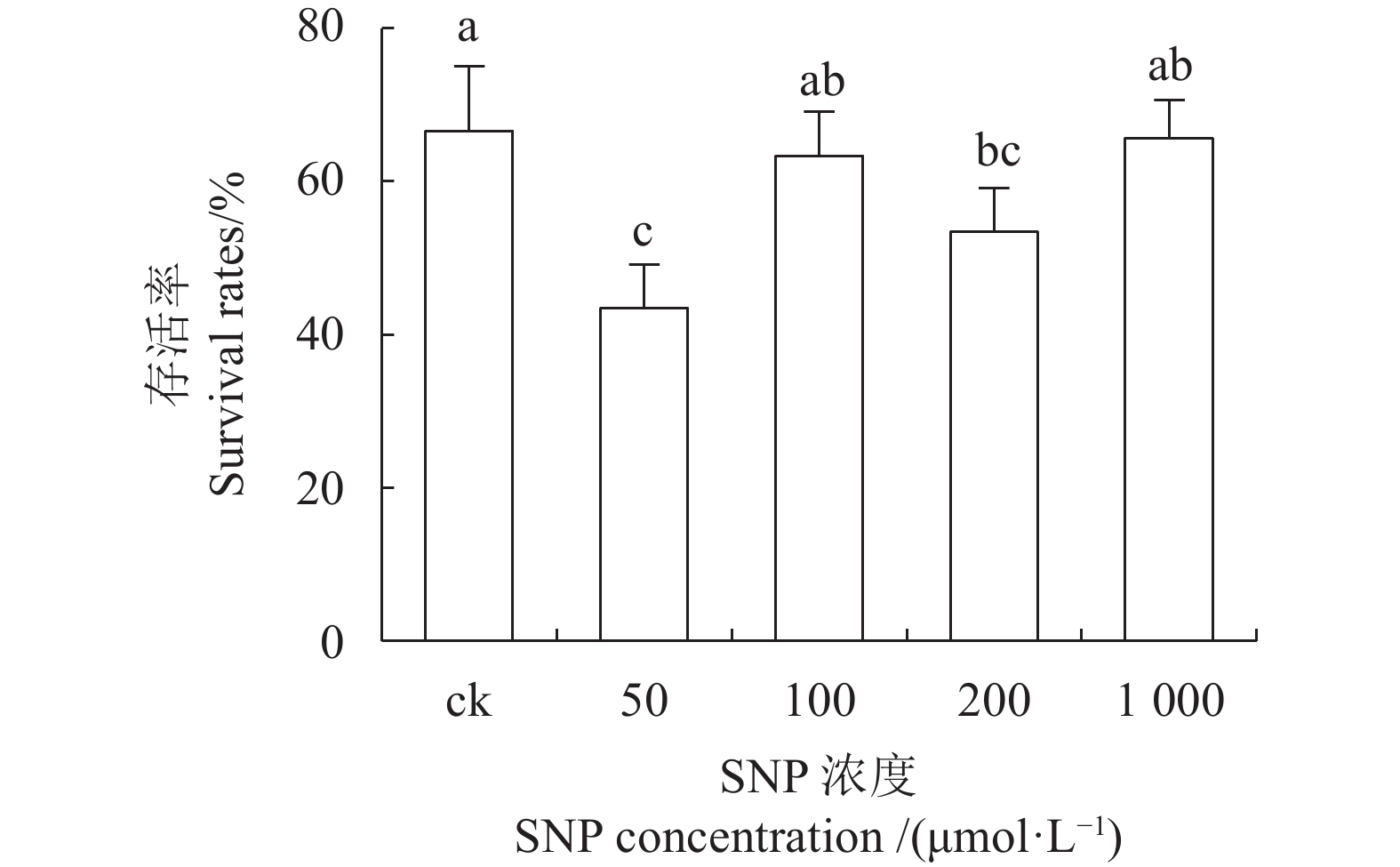
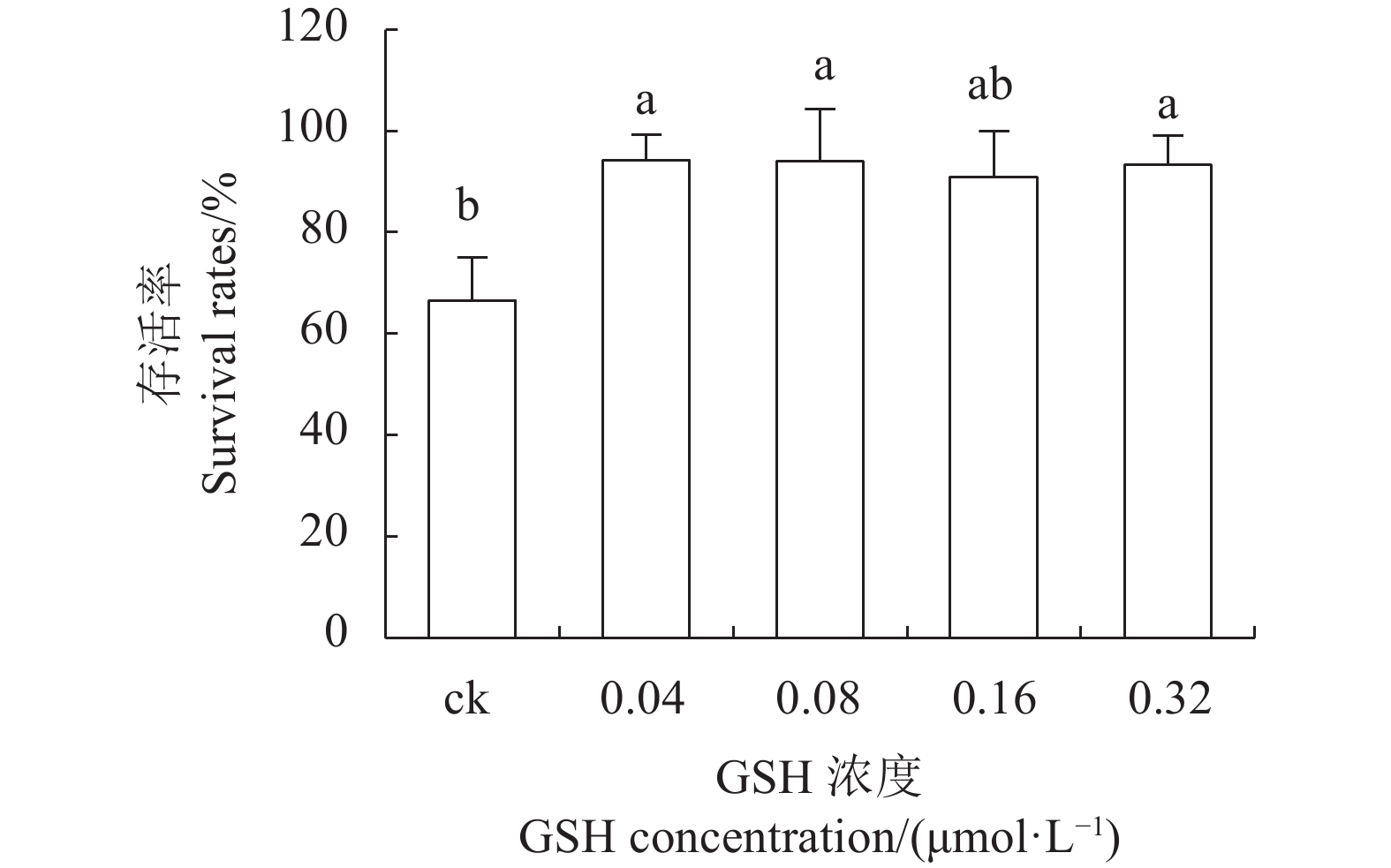
目的 建立红丽海棠超低温保存技术体系,实现其种质资源保存。 方法 以红丽海棠茎尖为试材,研究蔗糖预处理浓度和时间、装载处理时间、PVS2处理时间对其超低温保存后的存活率的影响,并在此基础上,在超低温保存程序中的不同环节添加3种抗氧化剂[过氧化氢酶(Catalase, CAT)、抗坏血酸(Ascorbic acid, AsA)和谷胱甘肽(Glutathione, GSH)]和2种细胞程序性死亡(PCD)抑制剂[(乙烯利Ethephon, Eth)和NO供体(Sodium Nitroprusside, SNP)],探讨其对茎尖冻后存活率的影响。 结果 (1)红丽海棠茎尖的玻璃化保存程序为:首先将红丽组培苗切为4~5 mm带顶芽茎段,接种在含0.7 mol·L−1蔗糖的预培养基后放入4 ℃冰箱冷锻炼2 d;再将其切成约1.5~2.0 mm的茎尖,置于Loading溶液中,在室温下处理20 min,随后于0 ℃下PVS2溶液处理90 min,立即投入液氮冻存;需用时将其从液氮罐取出后,快速放入38 ℃水浴中化冻1 min,再用Unloading溶液在室温下震荡洗涤2次,每次10 min。采用此方法红丽海棠茎尖液氮冻后存活率为66.58%,恢复生长率为16.67%。(2)不同浓度的外源添加剂对红丽海棠冻后存活率影响不同。其中,CAT、AsA和GSH最佳添加浓度分别为200 U·ml−1、400 μmol·L−1和0.04 μmol·L−1,较对照冻后存活率分别提高了20.28%、6.75%和27.61%,添加Eth和SNP没有显示明显的正向作用。 结论 红丽海棠茎尖可以实现超低温保存,外源添加适宜浓度GSH、CAT、AsA可以提高液氮冻存后率,效果最好的GSH可将恢复生长率从16.67%提高到41.39%。 Abstract:Objective Cryopreservation technology was applied for the conservation of the ornamental Malus Red Splendor crabapple germplasm. Methods Sucrose concentration and time for pretreatment as well as conditions for the subsequent loading, PVS2, and unloading treatments on the shoot tips of Malus spp. were studied. Under the selected preservation conditions, additional improvement on the post-freezing survival of the shoots by application of antioxidants (i.e., CAT, AsA, and GSH) and PCD inhibitors (i.e., Eth and SNP) was explored. Results (1) The procedures employed for vitrification to preserve the shoots included cutting the histoponic seedlings into 4-5 mm segments containing terminal buds, inoculating the cut stems segments in a pre-culture medium containing 0.7 mol·L−1 sucrose at 4 ℃ for 2 d, cutting the tips into approximately 1.5-2 mm in length and placing in a loading solution for 20 m at room temperature, treating with PVS2 solution for 90 m at 0 ℃ followed by immediate submerging in liquid nitrogen, removing the cryogenically frozen tips to rapidly thaw them in a water bath at 38 ℃ for 1 m, and rinsing twice with an unloading solution at room temperature with constant shaking for 10 m each time. The post-cryopreservation survival rate of the shoot tips was 66.58%, and 16.67% of them recovered to grow as normal. (2) The best improvement by the addition of various agents was found to be CAT at 200U·mL−1, AsA at 600 μmol·L−1, and GSH at 0.04 μmol·L−1, which increased the post-freezing survival rate by 20.28%, 6.75%, and 27.61%, respectively, over control. On the other hand, the addition of Eth or SNP posted no significant effect on the tip survival. Conclusion The survival rate of the post-cryopreservation Malus Red Splendor shoot tips could be further improved by the addition of GSH, CAT or AsA in appropriate concentration. GSH could most significantly raise the rate of viable plants after the cryopreservation from 16.67% to 41.39%. -
Key words:
- Ornamental crabapple /
- cryopreservation /
- antioxidants /
- programmed cell death
-
图 1 茎尖玻璃化超低温保存及存活率检测
A.红丽海棠组培苗;B.预培养;C.切取1.5~2.5 mm茎尖;D.茎尖在离心管中;E.经TTC染色变红(左)与未染色(右)的茎尖;F.冻后茎尖接种到恢复培养基中。
Figure 1. Survival rate of post-cryopreservation shoot tips
A: Crabapple tissue culture; B: pre-culture; C: 1.5-2.5 mm cut of shoot tip; D: shoot tip segment in centrifugal tube; E: TTC stained red (left) and original (right) shoot tips; F: post-frozen shoot tips inoculated on recovery medium.
-
[1] 王越, 刘燕. 观赏植物种质资源的超低温保存 [J]. 植物生理学通讯, 2006, 42(3):559−566.WANG Y, LIU Y. Cryopreservation of ornamental plant germplasm [J]. Plant Physiology Communications, 2006, 42(3): 559−566.(in Chinese) [2] JIA M X, JIANG X R, XU J, et al. CAT and MDH improve the germination and alleviate the oxidative stress of cryopreserved Paeonia and Magnolia pollen [J]. Acta Physiologiae Plantarum, 2018, 40(2): 37. doi: 10.1007/s11738-018-2612-0 [3] DI W, JIA M X, XU J, et al. Exogenous catalase and pyruvate dehydrogenase improve survival and regeneration and affect oxidative stress in cryopreserved Dendrobium nobile protocorm-like bodies [J]. Cryo Letters, 2017, 38(3): 228−238. [4] 狄玮, 贾梦雪, 刘燕. 外源抗氧化剂对春石斛类原球茎超低温保存的影响 [J]. 植物生理学报, 2015, 51(11):1880−1886. doi: 10.13592/j.cnki.ppj.2015.0335DI W, JIA M X, LIU Y. Effects of exogenous antioxidants on cryopreservation of protocorm-like bodies of Dendrobium nobile [J]. Plant Physiology Journal, 2015, 51(11): 1880−1886.(in Chinese) doi: 10.13592/j.cnki.ppj.2015.0335 [5] CHEN G Q, REN L, ZHANG D, et al. Glutathione improves survival of cryopreserved embryogenic calli of Agapanthus praecox subsp. orientalis [J]. Acta Physiologiae Plantarum, 2016, 38(10): 250. doi: 10.1007/s11738-016-2271-y [6] 张洁. ROS诱导的氧化胁迫与细胞凋亡对百子莲愈伤组织超低温保存细胞活性的影响机制[D]. 上海: 上海交通大学, 2015.ZHANG J. Influence mechanism of ROS induced oxidative stress and apoptosis on cell viability of Agapanthus praecox callus during cryopreservation[D]. Shanghai: Shanghai Jiao Tong University, 2015. (in Chinese) [7] 姜雪茹. 春石斛类原球茎玻璃化超低温保存中程序性细胞死亡研究[D]. 北京: 北京林业大学, 2019.JIANG X R. Study on the mechanism of programmed cell death during vitrification-cryopreservation in Dendrobium protocorm-like bodies[D]. Beijing: Beijing Forestry University, 2019. (in Chinese) [8] 张玲玲. 乙烯在春石斛 PLBs 超低温保存 PCD 中的作用及其与 H2O2的关系研究[D]. 北京林业大学, 2021.ZHANG L L. The role of ethylene and its relationship with H2O2 in PCD during Dendrobium protocorm-like bodies cryopreservation[D]. Beijing Forestry University, 2021. (in Chinese) [9] 王敏瑞. 苹果带毒茎尖超低温保存与茎尖超低温技术对病毒脱除和保存效应的研究[D]. 杨凌: 西北农林科技大学, 2019.WANG M R. Cryopreservation of apple with virus infection, and assessments of shoot tip cryotechniques for virus eradication and preservation in apple[D]. Yangling: Northwest A & F University, 2019. (in Chinese) [10] 冯超红. 苹果茎尖超低温保存技术及脱毒效率的研究[D]. 杨凌: 西北农林科技大学, 2014FENG C H. Cryogenic techniquesfor germplasm conservation and virus eradication in apple (malus)[D]. Yangling: Northwest A & F University, 2014. (in Chinese) [11] 胡凌云. 苹果茎尖超低温保存与脱毒研究[D]. 杨凌: 西北农林科技大学, 2015HU L Y. Cryopreservation and viruse eradication in shoot tips of apple[D]. Yangling: Northwest A & F University, 2015. (in Chinese) [12] 李百荃. 苹果属(Malus)试管苗植株再生、茎尖超低温保存和脱毒技术研究[D]. 杨凌: 西北农林科技大学, 2016.LI B Q. Plant regeneration, cryopreservation and virus eradication of apple (Malus)[D]. Yangling: Northwest A & F University, 2016. (in Chinese) [13] 徐瑾. 玉兰花粉超低温保存机制研究[D]. 北京: 北京林业大学, 2014.XU J. A study on the mechanism of Magnolia denudata pollen cryopreservation[D]. Beijing: Beijing Forestry University, 2014. (in Chinese) [14] VANDENBUSSCHE B, DE PROFT M P. Cryopreservation of in vitro sugar beet shoot tips using the encapsulation-dehydration technique: Influence of abscisic acid and cold acclimation [J]. Plant Cell Reports, 1998, 17(10): 791−793. doi: 10.1007/s002990050484 [15] NIINO T, TASHIRO K, SUZUKI M, et al. Cryopreservation of in vitro grown shoot tips of cherry and sweet cherry by one-step vitrification [J]. Scientia Horticulturae, 1997, 70(2/3): 155−163. [16] LIU Y G, WANG X Y, LIU L X. Analysis of genetic variation in surviving apple shoots following cryopreservation by vitrification [J]. Plant Science, 2004, 166(3): 677−685. doi: 10.1016/j.plantsci.2003.11.003 [17] KUSHNARENKO SVETLANA V, ROMADANOVA NATALIA V, REED BARBARA M. Cold acclimation improves regrowth of cryopreserved apple shoot tips [J]. Cryo Letters, 2009, 30(1): 47−54. [18] NIINO T, SAKAI A, YAKUWA H, et al. Cryopreservation of in vitro-grown shoot tips of apple and pear by vitrification [J]. Plant Cell, Tissue and Organ Culture, 1992, 28(3): 261−266. doi: 10.1007/BF00036122 [19] LIU Y G, LIU L X, WANG L, et al. Determination of genetic stability in surviving apple shoots following cryopreservation by vitrification [J]. Cryoletters, 2008, 29(1): 7−14. [20] HALMAGYI A, DELIU C, ISAC V. Cryopreservation of Malus cultivars: Comparison of two droplet protocols [J]. Scientia Horticulturae, 2010, 124(3): 387−392. doi: 10.1016/j.scienta.2010.01.012 [21] HALMAGYI A, VĂLIMĂREANU S, COSTE A, et al. Cryopreservation of Malus shoot tips and subsequent plant regeneration [J]. Romanian Biotechnological Letters, 2010, 15: 79−85. [22] 刘云国. 苹果种质资源玻璃化法超低温保存技术及其遗传变异分析[D]. 泰安: 山东农业大学, 2002.LIU Y G. Cryopreservation technique of apple germplasm by vitrification and analysis of genetic variation[D]. Taian: Shandong Agricultural University, 2002. (in Chinese) [23] URAGAMI A, SAKAI A, NAGAI M, et al. Survival of cultured cells and somatic embryos of Asparagus officinalis cryopreserved by vitrification [J]. Plant Cell Reports, 1989, 8(7): 418−421. doi: 10.1007/BF00270083 [24] 张晓丽, 吕萌, 马明倩, 等. ‘怀黄’菊微滴玻璃化超低温保存体系优化及保存后再生植株的抗冷性分析 [J]. 植物生理学报, 2021, 57(7):1427−1436. doi: 10.13592/j.cnki.ppj.2019.0479ZHANG X L, LÜ M, MA M Q, et al. Optimization of droplet-vitrification cryopreservation system and analysis of cold resistance of regenerated plants in Chrysanthemum × morifolium ‘Huaihuang’ [J]. Plant Physiology Journal, 2021, 57(7): 1427−1436.(in Chinese) doi: 10.13592/j.cnki.ppj.2019.0479 [25] 刘婧. 蓝莓、苹果不定芽超低温保存新技术的建立[D]. 杨凌: 西北农林科技大学, 2018.LIU J. Developments of novel cryopreservation techniques for blueberry and apple adventitious buds[D]. Yangling: Northwest A & F University, 2018. (in Chinese) [26] 贾梦雪. 春石斛优良品种早期筛选、快繁及超低温保存研究[D]. 北京: 北京林业大学, 2017JIA M X. The early screening, micrapropagation and cryopreservation of elite cultivar from nobile-type Dendrobium[D]. Beijing: Beijing Forestry University, 2017. (in Chinese) [27] ALMEIDA R D, ZERVOUDAKIS L K H, DUARTE M F Jr, et al. 225 oxidative stress and membrane integrity of cryopreserved spermatozoa with vitamin c [J]. Reproduction, Fertility and Development, 2014, 26(1): 226. [28] 孙鑫博, 代小梅, 王怡杰, 等. 植物细胞程序性死亡研究进展 [J]. 生物技术通报, 2010(11):1−6. doi: 10.13560/j.cnki.biotech.bull.1985.2010.11.002SUN X B, DAI X M, WANG Y J, et al. Advances in programmed cell death in plants [J]. Biotechnology Bulletin, 2010(11): 1−6.(in Chinese) doi: 10.13560/j.cnki.biotech.bull.1985.2010.11.002 [29] JIAO J, ZHOU B G, ZHU X P, et al. Fusaric acid induction of programmed cell death modulated through nitric oxide signalling in tobacco suspension cells [J]. Planta, 2013, 238(4): 727−737. doi: 10.1007/s00425-013-1928-7 [30] MA W W, XU W Z, XU H, et al. Nitric oxide modulates cadmium influx during cadmium-induced programmed cell death in tobacco BY-2 cells [J]. Planta, 2010, 232(2): 325−335. doi: 10.1007/s00425-010-1177-y [31] PAN C L, YAO S C, XIONG W J, et al. Nitric oxide inhibits Al-induced programmed cell death in root tips of peanut (Arachis hypogaea L. ) by affecting physiological properties of antioxidants systems and cell wall [J]. Frontiers in Physiology, 2017, 8: 1037. doi: 10.3389/fphys.2017.01037 [32] HE H Y, HUANG W J, OO T L, et al. Nitric oxide suppresses aluminum-induced programmed cell death in peanut (Arachis hypoganea L. ) root tips by improving mitochondrial physiological properties [J]. Nitric Oxide, 2018, 74: 47−55. doi: 10.1016/j.niox.2018.01.003 -








 下载:
下载: